4-methoxybenzoyl chloride appears as an amber-colored crystalline solid. Melting point 72 °F. Corrosive to metals and skin. Vapors may cause serious burns to the eyes. Decompose with water or alcohol, soluble in acetone and benzene.
The hydrolysis of 4-Methoxybenzoyl chloride (OMe) generally occurs through a dissociative mechanism (SN1). The reaction rate is strongly affected by the polarity of the medium and the ability to stabilize the leaving group. Water encapsulated within a reverse micellar system is less polar and less
available compared to a continuous aqueous phase; Consequently, the hydrolysis of OMe in the micellar medium is slower
than in pure water.
4-Methoxybenzoyl chloride is used in the synthesis of stilbene and dihydrostilbene derivatives as potential anti-cancer agents. It is also used in the synthesis of coumarin dimers with potential HIV-1 activity.
4-Methoxybenzoyl chloride is one of the reactive acylating agents that can react with carboxylic acids, alcohols and amines to yield respective carboxylic anhydrides, esters and amides.
4-Methoxybenzoyl chloride can be used as radical precursor in visible-light photocatalysis to synthesize various heterocyclic compounds.
It can be used to synthesize acylphosphine ligands for the rhodium-catalyzed hydrosilylation of alkenes.
Incorporation of 4-methoxybenzoyl chloride modified indium tin oxide (ITO) as cathode for the fabrication of organic light-emitting diodes (OLEDs) has been reported.
1,3 diketones synthesized from 4-methoxybenzoyl chloride can be used in one pot synthesis of various pyrazole derivatives.
It can also be used in the total synthesis of bioactive compounds like echinoside A and salinosporamide A.
4-Methoxybenzoyl chloride is synthesized from the direct chlorination of 4-methoxybenzoic acid by thionyl chloride.
4-methoxybenzoyl chloride appears as an amber-colored crystalline solid. Melting point 72°F. Corrosive to metals and skin. Vapors may cause serious burns to the eyes.
Fumes in air. Reacts exothermically with water (including moisture in air or soil) to form hydrochloric acid and insoluble anisic acid [Merck 11th ed. 1989].
ANISOYL CHLORIDE reacts exothermically with bases, including amines. Incompatible with water, strong oxidizing agents, alcohols. Sealed containers held at room temperature may explode, due to slow decomposition that builds up pressure. This situation is more dangerous with heat. May react vigorously or explosively with diisopropyl ether or other ethers in the presence of trace amounts of metal salts [J. Haz. Mat., 1981, 4, 291].
Solutions corrosive to tissue. Explosion risk
when in closed containers due to pressure caused
by decomposition at room temperature.
Vapor irritates mucous membranes. Contact of liquid with eyes or skin causes severe irritation. Ingestion causes severe irritation of mouth and stomach.
Special Hazards of Combustion Products: Irritating hydrogen chloride fumes may be formed.
Flammability and Explosibility
Not classified
Reactivity with Water Reacts slowly to generate hydrogen chloride (hydrochloric acid). The reaction is not hazardous; Reactivity with Common Materials: Corrodes metal slowly; Stability During Transport: Stable; Neutralizing Agents for Acids and Caustics: Flush with water, rinse with sodium bicarbonate or lime solution; Polymerization: Not pertinent; Inhibitor of Polymerization: Not pertinent.
Corrosive to skin, eyes,
mucous membranes, and other tissue.Evolves HCl by hydrolysis. A storage
hazard; can explode spontaneously at room
temperature. When heated to
decomposition it emits toxic fumes of Cland
may explode.
Stored in a closed container can cause an explosion due to the pressure
caused by decomposition, should be stored at low temperature (5°C).
Structure and conformation
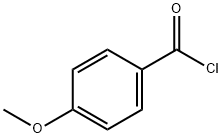
Anisoyl chloride, also known as methoxybenzoyl chloride), is an acyl halide, specifically an aromatic acyl chloride, and may be formed from anisic acid by replacing a hydroxyl group of the carboxylic acid with a chloride group. There are three isomers: the ortho-, meta-, and para- forms. 4-Methoxybenzoyl chloride is para- forms. Their structures differ in the arene substitution pattern—the location of the methoxy group on the ring as compared to the acyl halide.