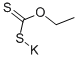
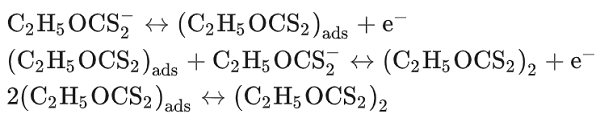Potassium ethylxanthate (KEtX) has long been utilized as a collector in the flotation of chalcocite (Cu2S), bornite (Cu5FeS4), chalcopyrite (CuFeS2) and galena (PbS). KEtX acts as an inhibitor of anodic corrosion of copper in acidic sodium chloride solutions. The collection efficiency of EtX? ion is ascribed to its interaction with mineral surfaces, possibly through a chemisorption bond. A similar interaction of EtX? ions with Pb has been found. It is interesting to note that the anodic oxidation of copper of KEtX to diethyl dixanthogen should be considered according to the following equations:
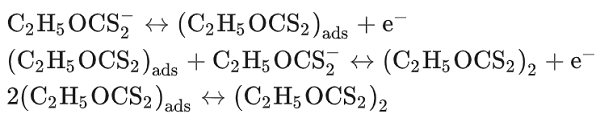
Diethyl dixanthoges does not diffuse into the bulk solution but forms a physically adsorbed layer. Interactions of EtX? ions with the Cu electrode surface are first manifested by the adsorption of EtX? ions, which is then followed, at higher positive potentials, by a growth of Cu(I)EtX film. On the other hand, the inhibitory effect of EtX? may probably be explained on the basis of stabilizing the +1 oxidation state of copper in the film against further oxidation and an increase in the hydrophobicity of the metal surface[1].