White crystalline solid with characteristic, very sweet, caramel-like
odor and taste. In dilute solution it possesses a sweet, fruitlike flavor
and odor.
Ethyl maltol has a very sweet, fruit-like odor of immense tenacity and sweet, fruity taste with initial bitter-tart flavor;
rapid loss of flavor per se. It is four to six times more potent than maltol.
It forms white crystals (mp 90–91°C) with very sweet
caramel-like odor, four to six times more potent than maltol.
Several syntheses have been developed for its preparation. In a one-pot process,
for example, -ethylfurfuryl alcohol is treated with halogen to give 4-halo-
6-hydroxy-2-ethyl-2H-pyran-3(6H)-one, which need not be isolated and can be
converted to ethylmaltol by aqueous hydrolysis
Has apparently not been reported to occur in nature.
In 1891, Bernhardi first discovered synthesis, and its derivatives are used for therapeutic purposes and can be prepared in industrial experiments. As war ammunition, it was first used by France in fortress warfare, and was prepared before the war. In October 1914, the French used it as a gas grenade, planned for the fortress battle. On January 7, 1915, it was actually used in the forest of Argonnen on the western front of France.
Ethyl Maltol is an extract from medicinal plants such as P. Incarnata and can be used as an anticonvulsant, acting as a depressant, and on motor activity.
Ethyl Maltol is a flavoring agent that is a white, crystalline powder.
it has a unique odor and a sweet taste that resembles fruit. the melt-
ing point is 90°c. it is sparingly soluble in water and propylene gly-
col and soluble in alcohol and chloroform. it is obtained by chemical
synthesis.
Flavor and fragrance enhancer in foods, especially baked goods, beverages, and synthetic berry and citrus flavorings; minimizes undesirable flavors in tobacco products, cough syrup, vitamins, cosmetics, and saccharin-containing products.
Several syntheses have been developed for its preparation. In a one-pot process,
for example, -ethylfurfuryl alcohol is treated with halogen to give 4-halo-
6-hydroxy-2-ethyl-2H-pyran-3(6H)-one, which need not be isolated and can be
converted to ethylmaltol by aqueous hydrolysis.
Unlike maltol, ethyl maltol does not occur naturally. It may be
prepared by treating a-ethylfurfuryl alcohol with a halogen to
produce 4-halo-6-hydroxy-2-ethyl-2H-pyran-3(6H)-one, which is
converted to ethyl maltol by hydrolysis.
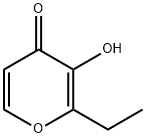
ChEBI: Ethyl maltol is a pyranone.
Fermentation method. Kojic acid is obtained from starch fermentation, and then ethyl maltol is obtained by etherification, oxidation, debenzylation, decarboxylation, hydroxylation and reduction.
Pyrofuroic acid method. A solution of pyrofuroic acid and acetic acid at a temperature of 90°C was added dropwise to the ether solution of diacetyl peroxide within 1~2h, and then the mixture was raised to 2h within 2h. 110 ° C, so that the 2-position of pyrofuroic acid can be directly alkylated to obtain ethyl maltol.
furfuryl alcohol method. Furfuryl alcohol is chlorinated in methanol aqueous solution by introducing chlorine gas to generate 4-chloro-3-hydroxy-4H-ketone, and then heated and hydrolyzed to obtain pyrofuroic acid; under alkaline conditions, pyrofuroic acid is condensed with acetaldehyde to obtain hydroxyethyl Pyrofuroic acid, which is reduced to ethyl maltol with zinc powder in hydrochloric acid.
Furfural method. Furfural reacts with ethylmagnesium bromide to obtain ethylfurfuryl alcohol (α-furan alkanol), which is then oxidized by chlorine gas in methanol aqueous solution at 0°C, and then heated to 100°C for hydrolysis to obtain ethyl maltol.
Taste characteristics at 70 ppm: sweet, burnt cotton, sugar candy-like with jamy, strawberry notes.
Ethyl maltol is a synthetic homologue of maltol, often found as flavor enhancers and it contributes to the fragrance of commercials products such as cereals, breads, malt beverages, coffee, soybeans and chocolate milk.
Flammability and Explosibility
Not classified
Pharmaceutical Applications
Ethyl maltol is used in pharmaceutical formulations and food
products as a flavoring agent or flavor enhancer in applications
similar to maltol. It has a flavor and odor 4–6 times as intense asmaltol. Ethyl maltol is used in oral syrups at concentrations of
about 0.004% w/v and also at low levels in perfumery.
Moderately toxic by
ingestion and subcutaneous routes.
Mutation data reported. When heated to decomposition it emits acrid smoke and
irritating fumes.
In animal feeding studies, ethyl maltol has been shown to be well
tolerated with no adverse toxic, reproductive, or embryogenic effects. It has been reported that while the acute toxicity of ethyl
maltol, in animal studies, is slightly greater than maltol, with
repeated dosing the opposite is true. The WHO has set an
acceptable daily intake for ethyl maltol at up to 2 mg/kg bodyweight.
LD50 (chicken, oral): 1.27 g/kg
LD50 (rat, oral): 1.15 g/kg
LD50 (mouse, oral): 0.78 g/kg
LD50 (mouse, SC): 0.91 g/kg
When orally administered, ethyl maltol was rapidly and extensively absorbed. Elimination was also extensive and rapid, involving conjugation as the glucuronide and ethereal sulphate, and excretion in the urine to the extent of 65-70% within 24 hr. Rate studies after iv dosage indicated that the bulk (86%) of the recovered conjugates was excreted within 6 hr (Rennhard, 1971).
Solutions may be stored in glass or plastic containers. The bulk
material should be stored in a well-closed container, protected from
light, in a cool, dry place.
GRAS listed. Included in the FDA Inactive Ingredients Database
(oral syrup).