Quinolones
Development of synthetic quinolone antibacterial agents has experienced three generations. The typical representative of the first generation quinolone is the nalidixic acid developed in 1962. It is effective in the treatment of Gram-negative bacteria such as Escherichia coli but not effective in the treatment in Pseudomonas aeruginosa and gram-positive bacteria with poor absorption capability and low biological availability. It is easy to cause bacteria drug resistance and thus had been eliminated. Typical representative of the secondary generation of quinolones include oxolinic acid and pipemidic acid developed in 1970s. In 1979, our country had applied pipemidic acid to the treatment of infections caused by Gram-negative bacteria and had achieved excellent efficacy. It therapeutic efficacy on Pseudomonas aeruginosa infection is superior to nalidixic acid and carbenicillin, but not as good as gentamicin. The disadvantage is that it has a poor efficacy in the treatment of gram-positive bacteria with low blood levels as well as certain central nervous system toxicity.
In the late 1970s, the development of cephalosporins had reached its peak. However, the price is so high that general patient can hardly afford it. The development of the third generation quinolone-synthetic fluoroquinolone antibiotic had given the world the feeling of “mountains multiply and streams double back no doubt, there is a way out". Fluoroquinolone antibiotic refers to the novel type of quinolone antibacterial drugs with the six position of the synthetic quinazoline ring being introduced into a fluorine atom. They not only have a 4-64 fold stronger efficacy against gram-negative bacteria than the first and second generation quinolones, but also have an 8 to 64 fold stronger effect than the first and second generation quinolones in the treatment of gram-positive bacteria. Moreover, they have excellent oral absorption property and rarely cause drug resistance. Furthermore, as a kind of synthetic chemicals, it is relatively more inexpensive than general antibiotics (especially third generation cephalosporins).
The DNA of bacterial cells exists in the form of double-stranded helix with the formation of double helix relying on the action of DNA gyrase. The mechanism of action of quinolones is through the inhibition of DNA gyrase, causing chromosome damage, resulting in the failure of the division and reproduction of the cells. Because of its unique mechanism of action, which is free of the impact from the plasmid conducting drug resistance, it has no cross-resistance with many kinds of antibacterial drugs.
The advantage of fluoroquinolone antibiotics are as follows:
1. It has broad antimicrobial spectrum and strong antibacterial activity with some of them (such as ofloxacin) having their effects comparable with third-generation cephalosporins. It also has effect against Gram-positive bacteria Staphylococcus aureus and refractory methicillin-resistant Staphylococcus aureus (MRSA); in terms of its antibacterial effect against gram-negative bacteria, it has spread to Pseudomonas aeruginosa, Haemophilus influenzae, and penicillin enzyme-producing Neisseria gonorrhoeae; some kinds of novel fluoroquinolone antibiotics are even effective in the treatment of mycoplasma and chlamydia.
2. It has excellent oral absorption property with wide tissue distribution. Usually fluoro quinolone administrated orally need 1 to 2 hours to reach the concentration peak in blood. It has a low plasma protein binding rate at about 10% to 40%. After the drug administration, it can be widely distributed in liver, kidney, skin and lungs and other tissues.
3. It has wide range of treatment with certain therapeutic effect on intestinal, urinary tract, biliary tract, respiratory tract infections, prostatitis, osteomyelitis, etc. It is widely used in the treatment of infections of various subjects.
4. Low incidence of drug resistance. Ofloxacin, used in Germany, can still inhibit over 96.8% of Gram-negative bacteria and 93.3% of gram-positive bacteria after eight years; ciprofloxacin used in the UK has 91% of Pseudomonas aeruginosa and 95% of Staphylococcus be sensitive to it; However, there is research in our country indicated that the resistance against fluoroquinolone for Pseudomonas aeruginosa has risen in recent years, from 4.4% to 10%.
Characteristics of fluoroquinolone antibiotics are as follows.
1. Its antibacterial effect, in general, is poorer against gram-positive bacteria than gram-negative bacteria.
2. The related adverse effects include gastrointestinal symptoms, usually doesn’t need stopping; for central nervous system symptoms, such as anxiety, nervousness, insomnia and headaches are more common for ciprofloxacin; rash may also occur; the incidence of liver and kidney dysfunction is generally 0.5% to 1.0%.
3. Long-term puppy magnetic resonance imaging (MRI) and ultrasound studies have shown that there is loose phenomenon occurring on the intermediate layer of bone and joint cartilage matrix. However, this phenomenon hasn’t been observed in hundreds of cases of human. Still for safety purpose, this class of drugs is not recommended to be long-term administrated to lactating woman and children upon bone development in large dose. Because this product inhibit the replication of DNA, so pregnant women should also administrate it with caution.
4. Individual kinds of fluoroquinolones (such as lomefloxacin, enoxacin and sparfloxacin), when being subject to long-term administration to elderly outdoor-working farmers in large dose, can cause phototoxic reactions in a few cases.
There are several conditions where drug interactions can happen:
1. administration together with milk, cheese and calcium, magnesium, iron, aluminum and other drugs will affect the antibacterial effect; it is generally recommended to be administrated upon an empty stomach; if administrated after meals, the peak time of drug in the blood will be postponed for 1 to 2 hours, but the total amount of the absorption will not be affected; acidic urine can further facilitate its excretion while precipitation can happen in alkaline urine.
2. Interaction with theophylline and other xanthine drugs can be divided into three categories: when administrated together with enoxacin, the plasma concentration of theophylline can be increased by two fold; administration together with tosufloxacin and ciprofloxain can cause 20% increase of the blood concentration of theophylline; administration together with lomefloxacin and norfloxacin has no effect on plasma concentrations of theophylline.
3. Administration together with non-steroidal anti-inflammatory drugs fenbufen can promote the binding between fenbufen and γ- aminobutyric acid (GABA) receptors to induce epileptic seizures, so patients with a history of epilepsy should administrate with caution.
4. Administration together with other antimicrobial agents should also be cautious. For example, combination with vancomycin can cause increase in renal toxicity; combination with doxorubicin, furadantin can cause decreased kidney function (ciprofloxacin); combination with chloramphenicol, doxycycline, clindamycin and macrolide antibiotic can even cause decrease of its antibacterial effect, and can also prone to lead to adverse reactions of hematopoietic system and the nervous system.
- Structure:
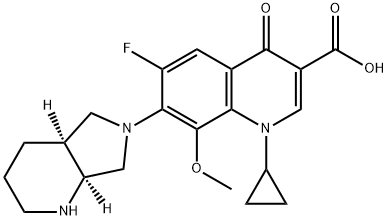
- Chemical Name:Moxifloxacin
- CAS:151096-09-2
- MF:C21H24FN3O4
- Structure:
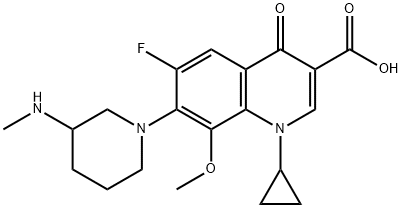
- Chemical Name:Balofloxacin
- CAS:127294-70-6
- MF:C20H24FN3O4
- Structure:
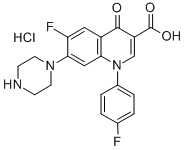
- Chemical Name:Sarafloxacin hydrochloride
- CAS:91296-87-6
- MF:C20H18ClF2N3O3
- Structure:
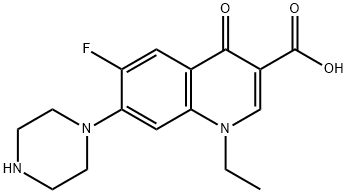
- Chemical Name:Norfloxacin
- CAS:70458-96-7
- MF:C16H18FN3O3
- Structure:
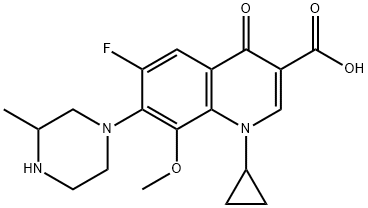
- Chemical Name:Gatifloxacin
- CAS:112811-59-3
- MF:C19H22FN3O4
- Structure:
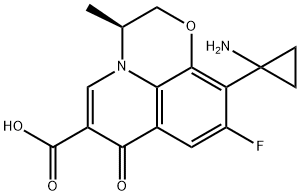
- Chemical Name:Pazufloxacin
- CAS:127045-41-4
- MF:C16H15FN2O4
- Structure:
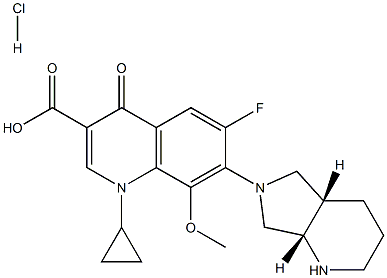
- Chemical Name:Moxifloxacin hydrochloride
- CAS:186826-86-8
- MF:C21H25ClFN3O4
- Structure:
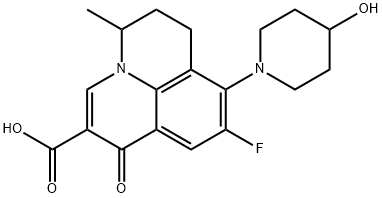
- Chemical Name:Nadifloxacin
- CAS:124858-35-1
- MF:C19H21FN2O4
- Structure:
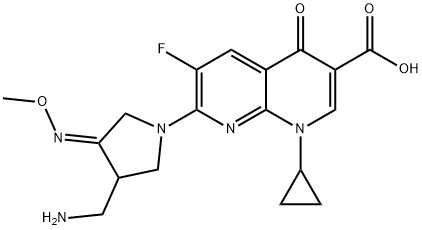
- Chemical Name:Gemifioxacin
- CAS:175463-14-6
- MF:C18H20FN5O4
- Structure:
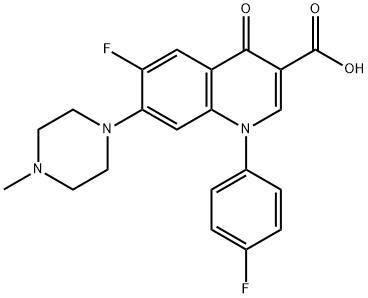
- Chemical Name:Difloxacin
- CAS:98106-17-3
- MF:C21H19F2N3O3
- Structure:
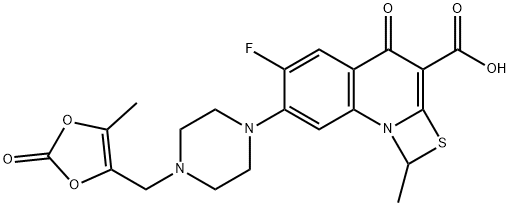
- Chemical Name:Prulifloxacin
- CAS:123447-62-1
- MF:C21H20FN3O6S
- Structure:
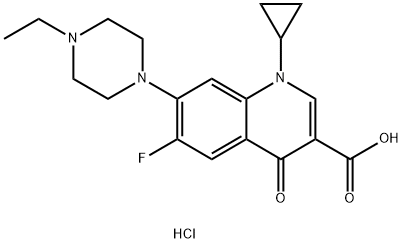
- Chemical Name:Enrofloxacin hydrochloride
- CAS:112732-17-9
- MF:C19H23ClFN3O3
- Structure:
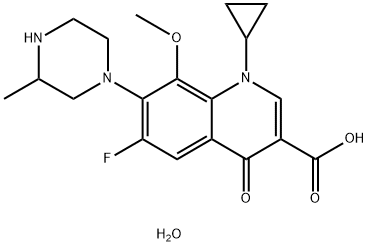
- Chemical Name:Gatifloxacin sesquihydrate
- CAS:180200-66-2
- MF:C19H24FN3O5
- Structure:
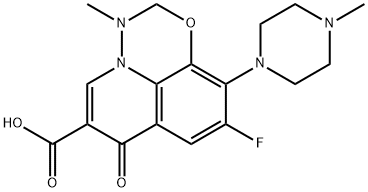
- Chemical Name:Marbofloxacin
- CAS:115550-35-1
- MF:C17H19FN4O4
- Structure:

- Chemical Name:Pazufloxacin mesilate
- CAS:163680-77-1
- MF:C17H19FN2O7S
- Structure:
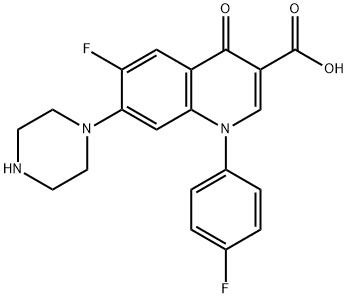
- Chemical Name:Sarafloxacin
- CAS:98105-99-8
- MF:C20H17F2N3O3
- Structure:
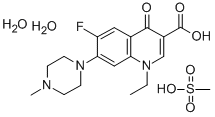
- Chemical Name:1-ETHYL-6-FLUORO-7-(4-METHYLPIPERAZIN-1-YL)-4-OXO-QUINOLINE-3-CARBOXYLIC ACID
- CAS:149676-40-4
- MF:C18H28FN3O8S
- Structure:
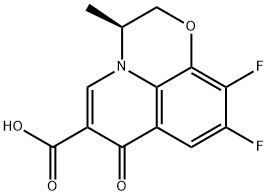
- Chemical Name:Levofloxacin carboxylic acid
- CAS:100986-89-8
- MF:C13H9F2NO4
- Structure:
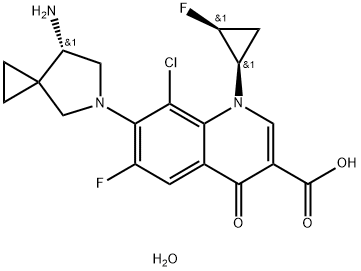
- Chemical Name:Sitafloxacin Sesquihydrate
- CAS:163253-35-8
- MF:C19H20ClF2N3O4
- Structure:
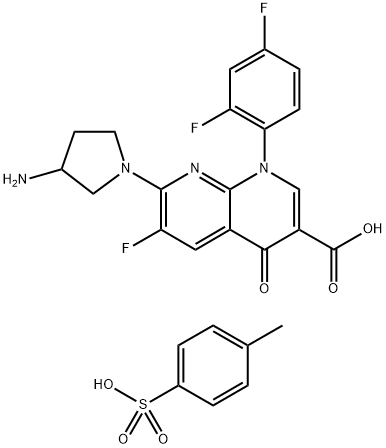
- Chemical Name:Tosufloxacin tosylate
- CAS:115964-29-9
- MF:C26H23F3N4O6S
- Structure:
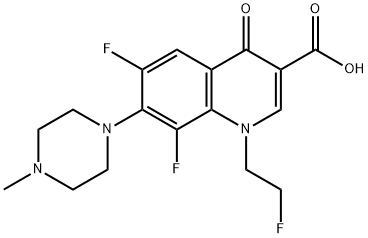
- Chemical Name:Fleroxacin
- CAS:79660-72-3
- MF:C17H18F3N3O3
- Structure:
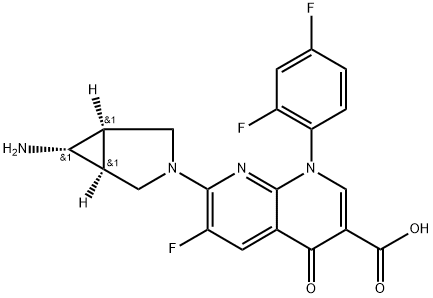
- Chemical Name:TROVAFLOXACIN
- CAS:147059-72-1
- MF:C20H15F3N4O3
- Structure:
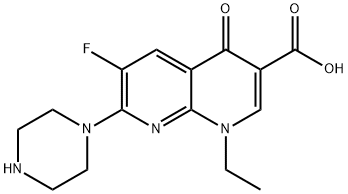
- Chemical Name:Enoxacin
- CAS:74011-58-8
- MF:C15H17FN4O3
- Structure:
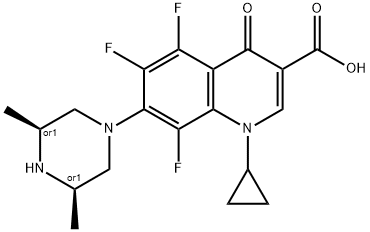
- Chemical Name:Orbifloxacin
- CAS:113617-63-3
- MF:C19H20F3N3O3
- Structure:
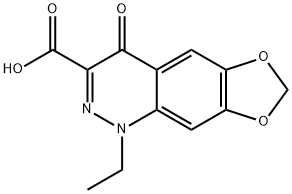
- Chemical Name:CINOXACIN
- CAS:28657-80-9
- MF:C12H10N2O5
- Structure:
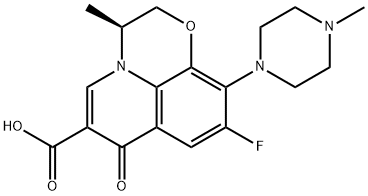
- Chemical Name:Levofloxacin
- CAS:100986-85-4
- MF:C18H20FN3O4
- Structure:
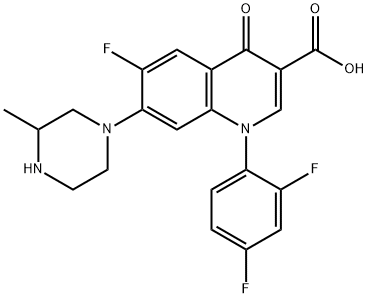
- Chemical Name:TEMAFLOXACIN
- CAS:108319-06-8
- MF:C21H18F3N3O3
- Structure:
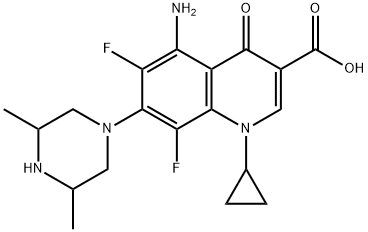
- Chemical Name:Sparfloxacin
- CAS:111542-93-9
- MF:C19H22F2N4O3
- Structure:
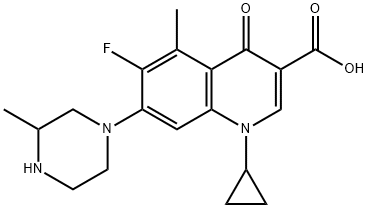
- Chemical Name:Grepafloxacin
- CAS:119914-60-2
- MF:C19H22FN3O3
- Structure:
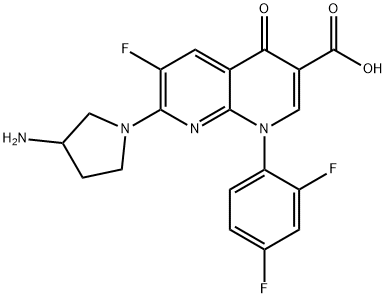
- Chemical Name:TOSUFLOXACIN TOSILATE
- CAS:100490-36-6
- MF:C19H15F3N4O3
- Structure:
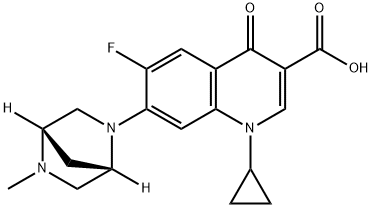
- Chemical Name:DANOFLOXACIN
- CAS:112398-08-0
- MF:C19H20FN3O3
- Structure:
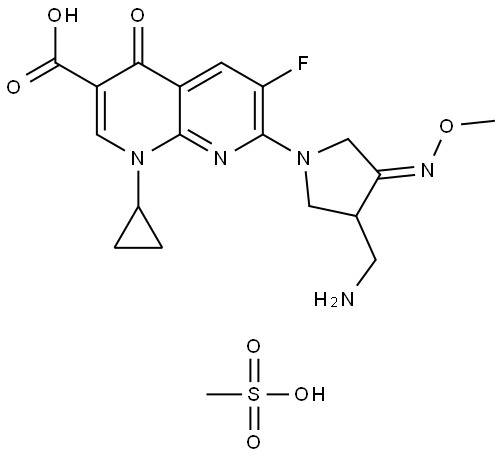
- Chemical Name:Gemifioxacin mesylate
- CAS:210353-53-0
- MF:C18H20FN5O4.CH4O3S
- Structure:
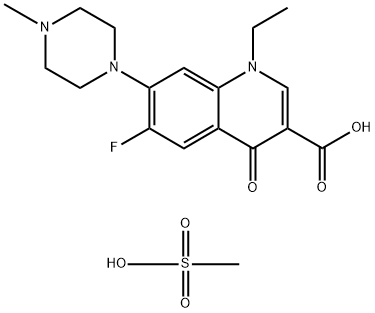
- Chemical Name:Pefloxacin mesylate
- CAS:70458-95-6
- MF:C18H24FN3O6S
- Structure:
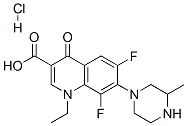
- Chemical Name:Lomefloxacin hydrochloride
- CAS:98079-52-8
- MF:C17H20ClF2N3O3
- Structure:
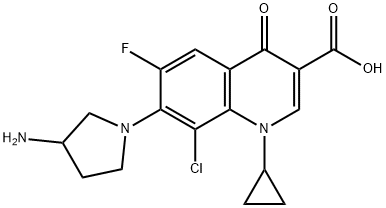
- Chemical Name:Clinafloxacin
- CAS:105956-97-6
- MF:C17H17ClFN3O3
- Structure:
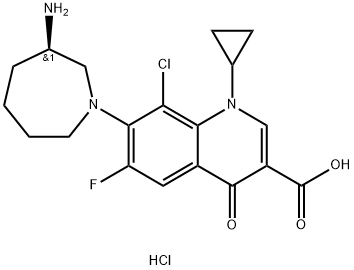
- Chemical Name:Besifloxacin hydrochloride
- CAS:405165-61-9
- MF:C19H22Cl2FN3O3
- Structure:
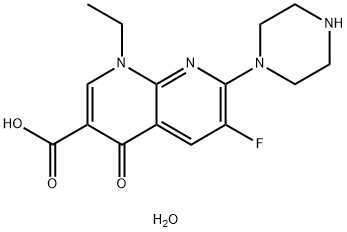
- Chemical Name:Enoxacin
- CAS:84294-96-2
- MF:C15H19FN4O4
- Structure:
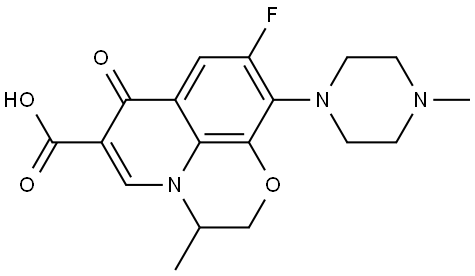
- Chemical Name:Ofloxacin
- CAS:82419-36-1
- MF:C18H20FN3O4
- Structure:
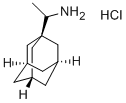
- Chemical Name:1-AdaMantanethylaMine
- CAS:13392-28-4
- MF:C12H21N
- Structure:
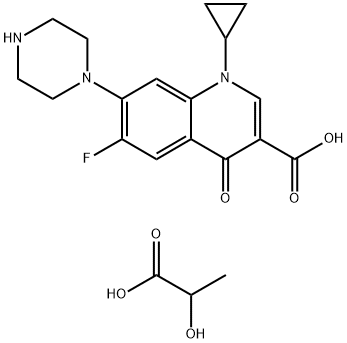
- Chemical Name:Ciprofloxacin lactate
- CAS:97867-33-9
- MF:C20H24FN3O6
- Structure:
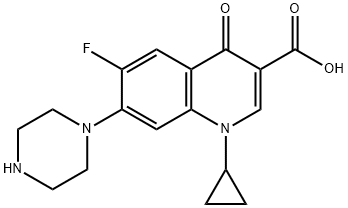
- Chemical Name:Ciprofloxacin
- CAS:85721-33-1
- MF:C17H18FN3O3
- Structure:
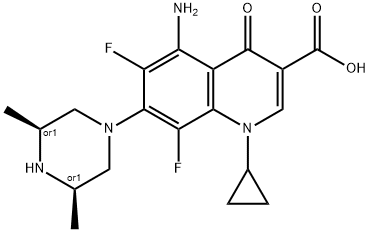
- Chemical Name:Sparfloxacin
- CAS:110871-86-8
- MF:C19H22F2N4O3
- Structure:
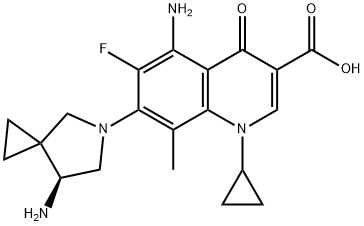
- Chemical Name:Olamufloxacin
- CAS:167887-97-0
- MF:C20H23FN4O3
- Structure:
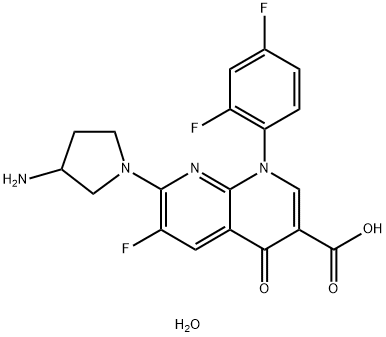
- Chemical Name:Tosufloxacin tosilate
- CAS:107097-79-0
- MF:C19H17F3N4O4
- Structure:
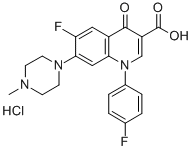
- Chemical Name:Difluoxacin hydrochloride
- CAS:91296-86-5
- MF:C21H20ClF2N3O3
- Structure:
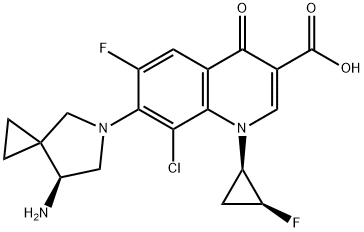
- Chemical Name:Sitafloxacin
- CAS:127254-12-0
- MF:C19H18ClF2N3O3
- Structure:
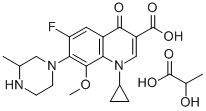
- Chemical Name:Gatifloxacin hydrochloride
- CAS:160738-57-8
- MF:C22H28FN3O7
- Structure:
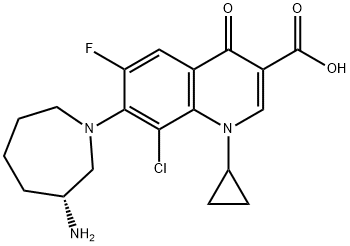
- Chemical Name:(R)-7-(3-Aminohexahydro-1H-azepin-1-yl)-8-chloro-1-cyclopropyl-6-fluoro-1,4-dihydro-4-oxo-3-quinolinecarboxylic acid
- CAS:141388-76-3
- MF:C19H21ClFN3O3
- Structure:
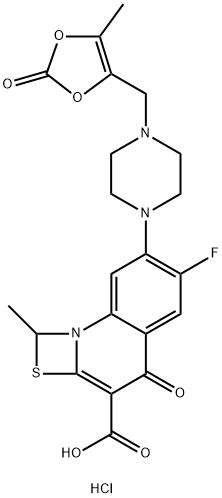
- Chemical Name:PRULIFLOXACIN
- CAS:123447-63-2
- MF:C21H20FN3O6S
- Structure:
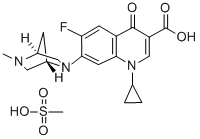
- Chemical Name:Danofloxacin mesylate
- CAS:119478-55-6
- MF:C20H24FN3O6S
- Structure:
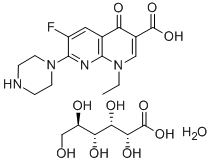
- Chemical Name:ENOXACIN GLUCONATE
- CAS:104142-71-4
- MF:C15H17FN4O3.C6H12O7.H2O
- Structure:

- Chemical Name:OFLOXACIN HYDROCHLORIDE
- CAS:
- MF:C18H21ClFN3O4
- Structure:
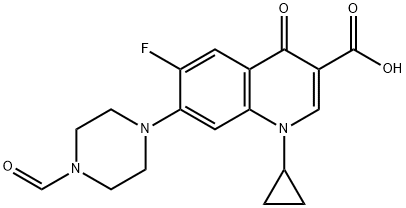
- Chemical Name:FORMYLCIPROFLOXACIN
- CAS:93594-39-9
- MF:C18H18FN3O4
- Structure:
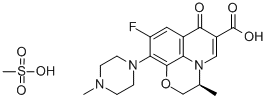
- Chemical Name:Levofloxacin mesylate
- CAS:226578-51-4
- MF:C19H24FN3O7S
- Structure:
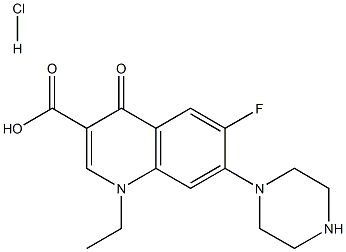
- Chemical Name:Norfloxacinehydrochloride
- CAS:68077-27-0
- MF:C16H19ClFN3O3
- Structure:
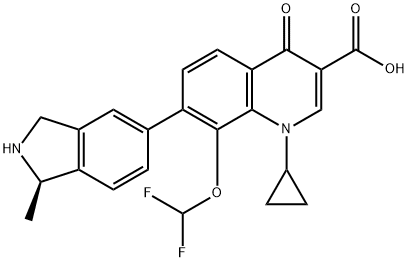
- Chemical Name:Garenoxacin
- CAS:194804-75-6
- MF:C23H20F2N2O4
- Structure:

- Chemical Name:LEVOFLOXACIN ACID ESTER
- CAS:
- MF:C15H13F2NO4
- Structure:

- Chemical Name:LEVOFLOXACIN ACID
- CAS:
- MF:C13H9F2NO4
- Structure:
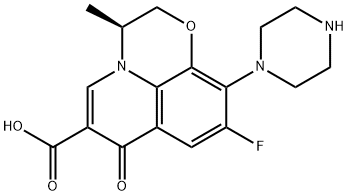
- Chemical Name:Desmethyl Levofloxacin
- CAS:117707-40-1
- MF:C17H18FN3O4
- Structure:
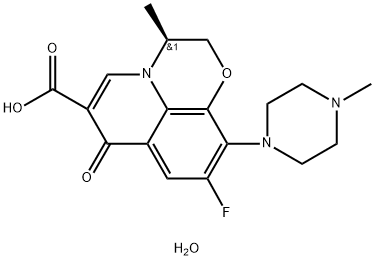
- Chemical Name:Levofloxacin heMihydrate
- CAS:138199-71-0
- MF:C18H22FN3O5
- Structure:
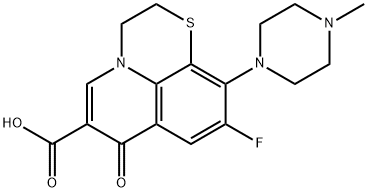
- Chemical Name:RUFLOXACIN
- CAS:101363-10-4
- MF:C17H18FN3O3S
- Structure:
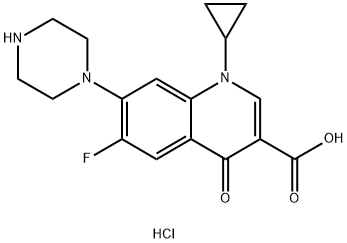
- Chemical Name:Ciprofloxacin hydrochloride
- CAS:86483-48-9
- MF:C17H18FN3O3.xHCl
- Structure:
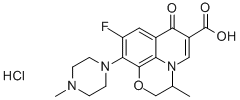
- Chemical Name:Ofloxacin hydrochloride
- CAS:118120-51-7
- MF:C18H20FN3O4.HCl
- Structure:
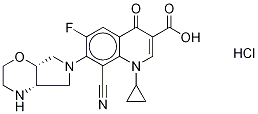
- Chemical Name:Finafloxacin Hydrochloride
- CAS:209342-41-6
- MF:C20H20ClFN4O4
- Structure:
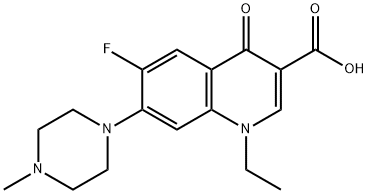
- Chemical Name:Pefloxacin
- CAS:70458-92-3
- MF:C17H20FN3O3
- Structure:
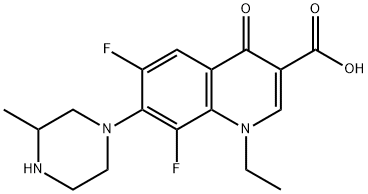
- Chemical Name:Lomefloxacin
- CAS:98079-51-7
- MF:C17H19F2N3O3
- Structure:
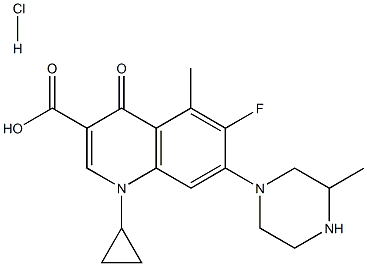
- Chemical Name:Grepafloxacin hydrochloride
- CAS:161967-81-3
- MF:C19H22FN3O3
- Structure:
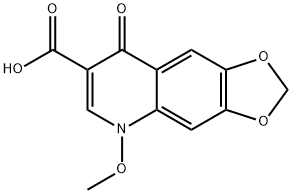
- Chemical Name:Miloxacin
- CAS:37065-29-5
- MF:C12H9NO6
- Structure:
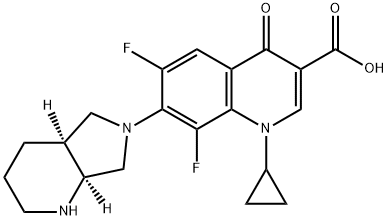
- Chemical Name:3-Quinolinecarboxylic acid, 1-cyclopropyl-6,8-difluoro-1,4-dihydro-7-(octahydro-6H-pyrrolo[3,4-b]pyridin-6-yl)-4-oxo-, (4aS-cis)-
- CAS:151213-15-9
- MF:C20H21F2N3O3
- Structure:
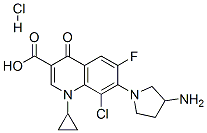
- Chemical Name:Clinafloxacin hydrochloride
- CAS:105956-99-8
- MF:C17H18Cl2FN3O3
- Structure:
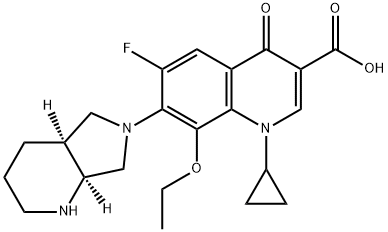
- Chemical Name:1-Cyclopropyl-8-ethoxy-6-fluoro-7-[(4aS,7aS)-octahydro-6H-pyrrolo[3,4-b]pyridin-6-yl]-4-oxo-1,4-dihydroquinoline-3-carboxylic acid
- CAS:1029364-75-7
- MF:C22H26FN3O4
- Structure:
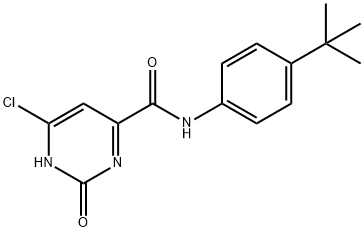
- Chemical Name:ENROFLOXACIN LACTATE
- CAS:931066-01-2
- MF:C15H16ClN3O2
- Structure:
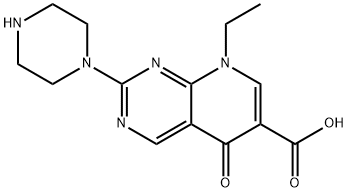
- Chemical Name:Pipemidic acid
- CAS:51940-44-4
- MF:C14H17N5O3
- Structure:
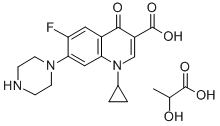
- Chemical Name:CIPROFLOXACIN LACTATE
- CAS:857213-31-1
- MF:C20H24FN3O6
- Structure:
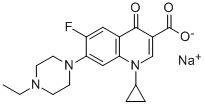
- Chemical Name:Enrofloxacin Sodium
- CAS:266346-15-0
- MF:C19H21FN3O3.Na
- Structure:
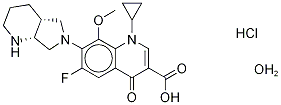
- Chemical Name:MOXIFLOXACIN, HYDROCHLORIDE MONOHYDRATE
- CAS:192927-63-2
- MF:C21H27ClFN3O5
- Structure:

- Chemical Name:OXOCIPROFLOXACIN
- CAS:
- MF:C17H16FN3O4
- Structure:
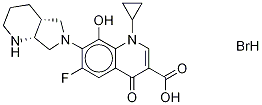
- Chemical Name:8-Hydroxy Moxifloxacin Hydrobromide
- CAS:
- MF:C20H23BrFN3O4
- Chemical Name:Alatrofloxacin
- CAS:
- MF:
- Structure:
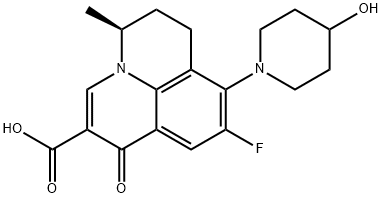
- Chemical Name:(S)-(-)-Nadifloxacin
- CAS:154357-42-3
- MF:C19H21FN2O4
- Structure:
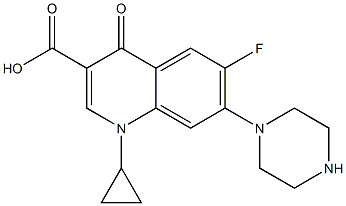
- Chemical Name:Ciprofloxacin IMpurity A
- CAS:
- MF:C17H18FN3O3
- Structure:
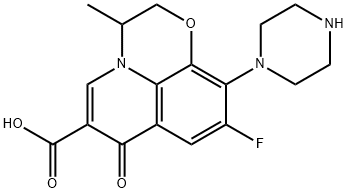
- Chemical Name:OFLOXACIN RELATED COMPOUND A (25 MG) ((RS)-9-FLUORO-2,3-DIHYDRO-3-METHYL-7-OXO-10-(PIPERA-ZIN-1 -YL)-7H-PYRIDO[1,2,3-DE]-1,4-BENZOXAZINE-6-CARBOXYLIC ACID)
- CAS:82419-52-1
- MF:C17H18FN3O4
- Structure:
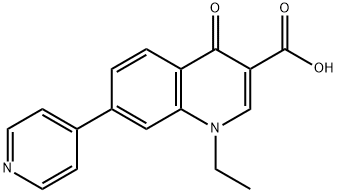
- Chemical Name:rosoxacin
- CAS:40034-42-2
- MF:C17H14N2O3
- Structure:
- Chemical Name:amifloxacin
- CAS:86393-37-5
- MF:C16H19FN4O3
- Structure:
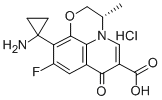
- Chemical Name:Pazufloxacin hydrochloride
- CAS:127046-45-1
- MF:C16H15FN2O4.HCl
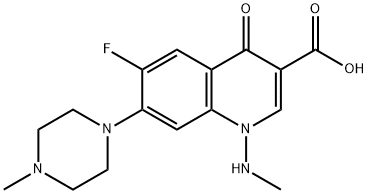
- Chemical Name:Gatifloxacin mesylate
- CAS:
- MF:
- Structure:
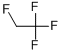
- Chemical Name:1,2,2,2-tetrafluoroethane
- CAS:29759-38-4
- MF:C16H18FN3O3C6H5NO3
- Chemical Name:SparfloxacinLactate
- CAS:
- MF:C19H22F2N4O3·C3H6O3
- Structure:
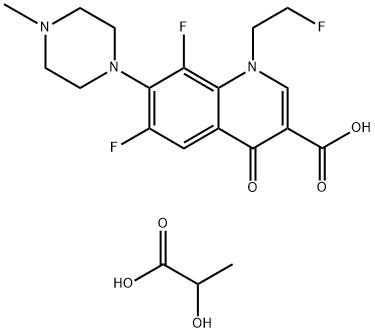
- Chemical Name:FLEROXACINLACTATE
- CAS:896139-71-2
- MF:C20H24F3N3O6
- Structure:
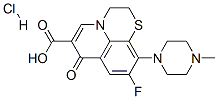
- Chemical Name:Rufloxacin hydrochloride
- CAS:106017-08-7
- MF:C17H19ClFN3O3S
- Chemical Name:levofloxacin/ofloxacin
- CAS:
- MF:
- Structure:
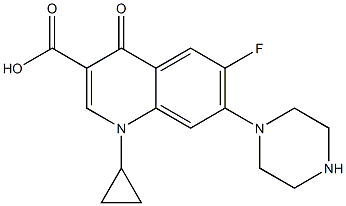
- Chemical Name:Ciprofloxacin IMpurity E
- CAS:
- MF:C17H18FN3O3
- Structure:
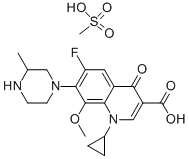
- Chemical Name:GATIFLOXACIN MESYLATE
- CAS:316819-28-0
- MF:C19H22FN3O4.CH4O3S
- Chemical Name:Lomefoxacin aspartate
- CAS:
- MF:
- Structure:
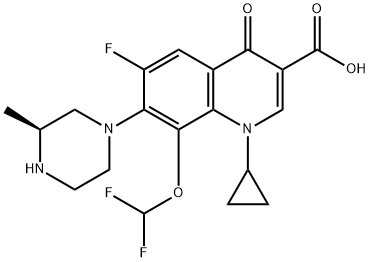
- Chemical Name:Cadrofloxacin
- CAS:153808-85-6
- MF:C19H20F3N3O4
- Structure:
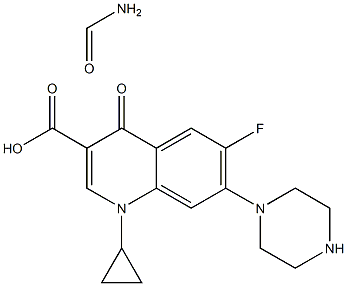
- Chemical Name:Ciprofloxacin Formamide
- CAS:
- MF:C18H21FN4O4
- Chemical Name:Enrofloxacin nicotinate
- CAS:
- MF:
- Structure:
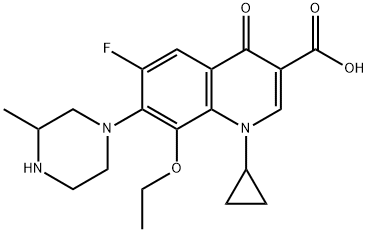
- Chemical Name:3-Quinolinecarboxylic acid, 1-cyclopropyl-8-ethoxy-6-fluoro-1,4-dihydro-7-(3-Methyl-1-piperazinyl)-4-oxo-
- CAS:182868-72-0
- MF:C20H24FN3O4
- Structure:

- Chemical Name:CIPROFLOXACIN IMPURITY A
- CAS:
- MF:C17H18FN3O3
- Structure:

- Chemical Name:Levofloxacin lactic acid
- CAS:
- MF:C21H24FN3O6