-
外観
無色~ほとんど無色, 澄明の液体
-
性質

三塩化リンの加水分解
三塩化リンには刺激臭があり、さまざまな金属を腐食します。融点は−112°C、沸点は74〜78°Cで、無色または黄色の発煙性液体です。
エーテル、ベンゼン、四塩化炭素などに可溶です。水と激しく反応し、塩化水素が発生して、ホスホン酸になります。三塩化リンはNH3でP(NH2)3に、ROHでP(OH)3に、O2で塩化ホスホリル (POCl3) に、Sで塩化チオホスホリル (PSCl3) になります。
-
溶解性
水と激しく反応, エーテル, ベンゼンに可溶。ジエチルエーテル、ベンゼン、二硫化炭素、四塩化炭素及びジクロロメタンに溶けやすい。
-
解説
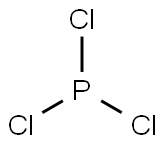
三塩化リンとは、化学式がPCl3で表されるリンの塩化物です。
三塩化リンは600ppmでも数分で死に至るほど、毒性や危険性が高いです。GHS分類で、眼刺激性、皮膚刺激性、急性毒性 (経口) 、特定標的臓器毒性 (単回、反復ばく露) に分類されています。
三塩化リンの法規制は、労働安全衛生法で名称等を表示・通知すべき危険物および有害物に指定されています。リスクアセスメントを実施すべき危険有害物に該当しており、毒物・劇物取締法では毒物です。
-
用途
医薬?農薬?染料?塩ビ安定剤原料,ドーピングガス
-
用途
有機合成原料。
-
構造
三塩化リンの分子量は137.33g/molで、密度は1.574g/cm3です。塩素原子の酸化数は−1価、リン原子の酸化数は+3価を取っています。
四塩化炭素中では0.8Dの双極子モーメントを有し、液体の標準生成エンタルピーは−319.7kJ/molです。三塩化リンの分子は、リン原子を頂点とした三方錐型構造を取っています。P-Clは2.039Åで、∠Cl-P-Clは100.27°です。
-
合成法
工業的には、黄リンや白リンにを吹き込んで得られる三塩化リン溶液を加熱還流して、三塩化リンを合成する方法が知られています。実験室では、毒性が低い赤リンを用います。
-
説明
Phosphorus trichloride, PCl3, is a clear, colorless, fuming, corrosive liquid. It decomposes rapidly in moist air and has a boiling point of about 168°F (75°C). PCl3 is corrosive to skin and tissue and reacts with water to form hydrochloric acid. The TLV is 0.2 ppm, and the IDLH is 50 ppm in air. The four-digit UN identification number is 1809. The NFPA 704 designation is health 4, flammability 0, and reactivity 2. The white section at the bottom of the diamond contains a W with a slash through it, indicating water reactivity. The primary uses are in the manufacture of organophosphate pesticides, gasoline additives, and dyestuffs; as a chlorinating agent; as a catalyst; and in textile finishing. Corrosives in contact with a poison may produce poison gases as the poison decomposes. In responding to an incident involving corrosives, the toxicity of the vapors could be much more of a concern for personnel than the corrosivity. When acids come in contact with cyanide, hydrogen cyanide gas, which is highly toxic, with a TLV of 10 ppm in air, is produced.
-
化学的特性
Phosphorus trichloride is a colorless to yellow, fuming liquid. Odor like hydrochloric acid.
-
物理的性質
Colorless fuming liquid; pungent odor; refractive index 1.516 at 14°C; density 1.574g/mL at 21°C; boils at 76°C; freezes at -112°C; decomposes in water; soluble in benzene, carbon disulfide, ether and chloroform and other halogenated organic solvents.
-
使用
Phosphorus trichloride is an important intermediate in the production of insecticides, herbicides, and organophosphorus pesticides as well as other chemicals such as phosphoryl chloride, phosphorus pentachloride, thiophosphoryl chloride, and phosphonic acid. It is also used in the production of synthetic surfactants, phosphites, gasoline additives, flame retardants, silver polish, and producing iridescent metallic deposits.
-
調製方法
Phosphorus trichloride (PCl3) is made by reacting yellow
phosphorus with chlorine and is used in chemical
manufacturing. It hydrolyzes to phosphoric acid and hydrochloric
acid.
-
反応プロフィール
Phosphorus trichloride is a strong reducing agent that may ignite combustible organic materials upon contact. May generate flammable and potentially explosive gaseous hydrogen upon contact with many common metals (except nickel and lead). Reactions with water are violent and produce heat and flashes of fire [AAR, 1999]. Gives intensely exothermic reactions with iodine monochloride [Mellor 2, Supp. 1:502. 1956]. Several laboratory explosions have been reported arising from mixtures with acetic acid, along with other acids, sulfuric acid and derivatives, carboxylic acids, etc. These have been ascribed to poor heat control allowing the formation of phosphine [J. Am. Chem. Soc. 60:488. 1938]. Ignites when mixed with hydroxylamine [Mellor 8:290. 1946-47]. Causes an explosion on contact with nitric acid [Comp. Rend. 28:86]. Phosphorus trichloride is incompatible with many common oxidants such as: sodium peroxide, fluorine, chromyl chloride, iodine chloride, to name a few. Isopropanol can react with PCl3, forming toxic HCl gas. (Logsdon, John E., Richard A. Loke., "Isopropyl Alcohol." Kirk-Othmer Encyclopedia of Chemical Technology. John Wiley & Sons, Inc. 1996.)
-
危険性
Phosphorus trichloride is highly corrosive. Its vapors are an irritant to mucous membranes. Chronic exposure to its vapors can cause bronchitis. It reacts violently with water and explodes in contact with acetic and nitric acids, and several other substances (Patnaik. P. 1999. A Comprehensive Guide to the Hazardous Properties of Chemical Substances, 2nd. Ed. New York: John Wiley & Sons).
-
健康ハザード
Phosphorus trichloride is highly toxic; it may cause death or permanent injury. Contact is highly irritating to the skin, eyes, and mucous membranes, and the material is an irritant through oral and inhalation exposure.
-
火災危険
Phosphorus trichloride will react violently with water, producing heat and toxic and corrosive fumes. When heated to decomposition, Phosphorus trichloride emits highly toxic fumes of chlorides and phosphorus oxides. Phosphorus trichloride may ignite other combustible materials. Reacts violently with water. Reacts explosively with acetic acid, aluminum, chromyl chloride, diallylphosphite and allyl alcohol, dimethyl sulfoxide, fluorine, hydroxylamine, iodine monochloride, lead dioxide, nitric acid, nitrous acid, organic matter, potassium, and sodium. Avoid contact with water, steam, or acids. Hazardous polymerization may not occur.
-
使用用途
三塩化リンの使用用途は幅広いです。例えば、難燃剤、酸化防止剤、可塑剤、殺虫剤、除草剤、水処理剤、界面活性剤などに用いられます。有機合成で有機ホスフィン配位子の導入剤や医薬中間体原料に使用可能です。
三塩化リンは、最も安価で多用途に用いられる3価のリン化合物です。そのため多くのリン化合物の出発原料に用いられています。難燃剤や可塑剤として広く利用される塩化ホスホリルのほか、医薬・有機合成の塩素化剤として広く用いられるなどの原料に利用されます。
-
安全性プロファイル
Poison by ingestion and
inhalation. A corrosive irritant to skin, eyes
(at 2 ppm), and mucous membranes.
Potentially explosive reaction with
chlorobenzene + sodtum, hethyl
sulfoxide, molten sodmm, chromyl chloride,
nitric acid, sodium peroxide, oxygen (above
100℃), tetravinyl lead. Reacts with
carboxylic acids (e.g., acetic acid) to form
violently unstable products. Violent reaction
or ignition with Al, chromium pentafluoride,
dtallyl phosphite + allyl alcohol, F2,
hexa fluoroisoprop ylideneaminolithium,
hydroxylamine, iodine chloride, PbO2,
HNO2, organic matter, potassium, selenium
dioxide, sulfur acids (e.g., sulfuric acid,
fluorosulfuric acid, oleum). Violent reaction
with water evolves hydrogen chloride and
diphosphane gas, that then ignite.
Incompatible with metals or oxidants. Wdl
react with water, steam, or acids to produce
heat and toxic and corrosive fumes; can
react with oxidzing materials. To fight fire,
use CO2, dry chemical. Used as a
chlorinating agent, catalyst, and chemical
intermedtate. Dangerous; when heated to
decomposition it emits highly toxic fumes of
Cland POx.
-
輸送方法
UN1809 Phosphorous trichloride, Hazard class: 6.1; Labels: 6.1-Poisonous materials, 8-Corrosive material, Inhalation Zone B.
-
純化方法
Heat it under reflux to expel dissolved HCl, then distil it. It has been further purified by vacuum fractionation several times through a -45o trap into a receiver at -78o. [Forbes Inorg Synth II 145 1946.] HARMFUL VAPOURS.
-
不和合性
Phosphorus trichloride is a strong reducing Violent reaction with water, producing heat and hydrochloric and phosphorous acids. Violent reaction with hydrides, alcohols, phenols and bases; water, when in contact with combustible organics; chemically active metals: sodium, potassium, aluminum; strong sulfuric or nitric acid. Attacks most metals except nickel and lead; may generate flammable hydrogen gas on contact with metals. Attacks plastics, rubber, and coatings.
-
廃棄物の処理
Decompose with water, forming phosphoric and hydrochloric acids. The acids may then be neutralized and diluted slowly to solution of soda ash and slaked lime with stirring, then flush to sewer with large volumes of water.