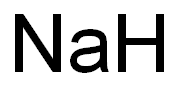-
溶解性
水と激しく反応して溶け、エタノールと反応して溶ける。
-
解説
ナトリウム,Na.原子番号11の元素.電子配置1s22s22p63s1の周期表1族元素.原子量22.99.安定同位体は 23Na だけであるが,ほかに6種類の放射性同位体が知られている.海水中には塩化ナトリウムとして約3% 含まれている.ナトリウムの水酸化物または塩化物の融解電解により得られる.真空蒸留により精製する.銀白色の軟らかい金属.等軸晶系.体心立方格子構造.格子定数a = 0.428 nm(20 ℃).融点97.8 ℃,沸点883 ℃.密度0.971 g cm-3(20 ℃).融解熱2.63 kJ mol-1.イオン化電位5.138 eV.炎色反応は黄色.空気中で酸化されてただちに光沢を失う.水とはげしく反応して水素を発生し,水酸化ナトリウムとなる.このとき,生成する反応熱のため水素が発火することもある.ハロゲン,酸素,ホウ素,炭素,ケイ素,硫黄族の元素とはげしく反応し,リン,ヒ素,水素とも化合する.アンモニアと反応しナトリウムアミドを生じる.高温では二酸化炭素,ケイ酸塩などとも反応し,ガラス,磁器を侵す.酸とは爆発的に反応する.塩においては酸化数1のイオンとして存在し,塩は一般に無色で,水に易溶で結晶水をもつものが多く,潮解性のものも少なくない.原子炉の冷却剤,ナトリウム化合物の製造,有機合成の還元剤,チタン・ジルコニウムなどの金属の製造,陰イオン重合触媒などに用いられる.
-
反応
ナトリウムは水と反応すると、水素が生じて水酸化ナトリウムになります。水素とともに加熱して、水素化ナトリウムを得ることも可能です。アルコール、フェノール、カルボン酸のヒドロキシ基と反応し、水素が発生して、アルコキシドなどを生成します。ナトリウムは単体のハロゲンとも反応し、塩を生成可能です。
ナトリウムは乾いた空気でもすぐに酸化して、酸化ナトリウムに変わり、金属光沢を失います。酸化ナトリウムを空気中に放置すると、二酸化炭素とも反応し、炭酸ナトリウムになります。
ナトリウムは還元剤として働くため、チタン、トリウム、タンタル、ジルコニウムのようなさまざまな金属を、容易に採取が可能です。
-
存在
ナトリウムは他のアルカリ金属元素と同様に反応性に富んでいるため、自然界で単体金属の状態をとることはできない。つねに1価の陽イオンとして化合物をつくり、地球上に広く分布している。海水中に、塩化ナトリウムに換算して平均2~3%の濃度で存在しているが、特別の場合として、ヨルダンとイスラエルにまたがる死海では20%もの濃度に達している。塩化ナトリウムはまた岩塩として巨大な鉱床をつくっている。炭酸塩(天然ソーダ)、硝酸塩(チリ硝石)、硫酸塩(ボウ硝)、ホウ酸塩(ホウ砂)なども鉱物として世界各地に産出する。また、不溶性のアルミノケイ酸塩、たとえば方沸石NaAlSi2O6・H2Oやソーダ長石NaAlSi3O8などにも含まれている。ナトリウムはまた動物体内に比較的多量に含まれ、組織液の浸透圧の維持や水素イオン濃度指数(pH)を一定に保つなどの重要な生理的役割を果たしている。
-
性質
ナトリウム,銀白色の軟らかい金属で、常温では体心立方構造をとっている。ナイフで切ったり、小孔から押し出して容易に針金状にすることができる。新しい面は金属光沢を呈するが、空気に触れるとただちに酸化されて光沢を失う。融点以上に熱すると炎をあげて燃える。ハロゲン、酸素などと激しく反応し、水素とも化合物をつくる。また水とも激しく反応して水素を発生し、反応熱のためにナトリウム自体は球状となって水面を走り回り、水酸化ナトリウムを生ずる。さらに発生した水素が空気と混合して爆発をおこす。したがって、保存するときは石油中に蓄えておく必要がある。ナトリウム塩は一般に水によく溶ける。
-
構造
ナトリウムは銀白色の柔らかい金属で、電子配置は[Ne] 3s1です。常温常圧では体心立方構造を取っていますが、200GPaの高圧下では結晶構造が変わり、金属光沢を失って透明になります。
ナトリウムには、20種の同位体が知られています。ただし、安定同位体は23Naだけです。それ以外は放射性同位体で、半減期が長いのは22Naと24Naであり、22Naの半減期は2.6年で、24Naの半減期は15時間です。22Naと24Naは、痕跡量が雨水などに含まれています。そのほかの放射性同位体は、すべて半減期が1分未満です。
ナトリウムには、2種類の核異性体が見つかっています。長寿命の核異性体には24mNaがあり、半減期は20.2ミリ秒です。
-
用途
シアン化ナトリウムの製造、過酸化ナトリウムの製造、染料、医薬品、有機合成
-
製法
工業的には融解塩を電気分解する方法によって製造されるが、原料として水酸化ナトリウムを用いるカストナー法と、塩化ナトリウムを用いるダウンズ法とがある。カストナー法では、鉄またはニッケルを陰極とし、黒鉛を陽極として320℃(水酸化ナトリウムの融点は318.4℃)付近で電解を行う。陰極では2Na++2e-―→2Na
ダウンズ法では、原料の塩化ナトリウムの融点(800.4℃)を下げるために塩化カリウムや塩化カルシウムを加える。こうすると600℃付近で電解が可能となる。
この方法では陽極で 2Cl-―→Cl2+2e-の反応がおこり、塩素が副産物として得られる。ダウンズ法では、塩化ナトリウムを水酸化ナトリウムに変えずに直接原料とすることができるうえ、電流効率も高く、塩素ガスが副生するなど多くの利点があるので、今日ではダウンズ法により多く製造されている。金属ナトリウムは減圧蒸留によって精製することができる。
-
説明
In its ionic form, sodium is one of the most important biological
nutrients and is found nearly everywhere on Earth.
Although it was isolated as a free metal in 1807 by Sir
Humphry Davy and makes up 2.83% of Earth’s lithosphere, it
is not found in its metallic form in nature. Pure sodium is
extremely reactive, particularly with water to form explosive
hydrogen gas and lye (NaOH); it can also react with water
vapor in air or biological tissues.
Mined and refined salts from terrestrial and aquatic sources
contain sodium in the form of sodium chloride, sodium
iodide, and other compounds. Natron, a naturally occurring
mixture of sodium compounds, has been used since the time of
the ancient Egyptians, and sodium compounds are essential to
numerous industries, including those involving glass, paper,
and soap production. Since it does not occur in its metallic
form in nature, pure sodium metal must be produced industrially,
which is accomplished via electrolysis of molten sodium
chloride.
-
化学的特性
Sodium is a soft silvery white metallic element. Pyrophoric solid or molten liquid. Odorless, oxidizing rapidly in air; waxlike at room temperature, brittle at low temperatures. Store in airtight containers or in naphtha or similar liquid that does not contain water or free oxygen. Decomposes water on contact, with evolution of hydrogen to form sodium hydroxide; insoluble in benzene, kerosene, and naphtha. Has excellent elec- trical conductivity and high heat-absorbing capacity.
-
物理的性質
Sodium is a soft, wax-like silver metal that oxidizes in air. Its density is 0.9674 g/cm3, andtherefore it floats on water as it reacts with the water releasing hydrogen. It has a rather lowmelting point (97.6°C) and a boiling point of 883°C. Sodium is an excellent conductor ofheat and electricity. It looks much like aluminum but is much softer and can be cut with aknife like butter. Its oxidation state is +1.
-
同位体
Sodium has 14 isotopes. The only stable isotope of sodium has an averageatomic weight of 23 (23Na) and makes up about 100% of all the isotopes of the element sodium found on Earth. All the other 13 isotopes (from 19Na to 31Na) are radioactive with relatively short half-lives and thus are unstable.
-
名前の由来
The Latin name for the symbol for “sodium” (Na) is natrium, and the
name “sodium” in Latin is sodanum, which was known as an ancient headache remedy
and was called “soda” in English.
-
天然物の起源
Sodium is the sixth most abundant of the Earth’s elements. Since it is a highly electropositive metal and so reactive with nonmetals, it is not found in its pure elemental form on Earth.Rather, it is found in numerous compounds in relatively abundant quantities. About 2.83%of the Earth’s crust consists of sodium in compounds.Sodium is produced by an electrolytic process, similar to the other alkali earth metals. (Seefigure 4.1). The difference is the electrolyte, which is molten sodium chloride (NaCl, common table salt). A high temperature is required to melt the salt, allowing the sodium cationsto collect at the cathode as liquid metallic sodium, while the chlorine anions are liberated aschlorine gas at the anode: 2NaCl (salt) + electrolysis → Cl2↑ (gas) + 2Na (sodium metal). Thecommercial electrolytic process is referred to as a Downs cell, and at temperatures over 800°C,the liquid sodium metal is drained off as it is produced at the cathode. After chlorine, sodiumis the most abundant element found in solution in seawater.
-
特性
On the periodic table sodium is located between lithium and potassium. A fresh cut intosodium looks silvery but turns gray as sodium oxidizes rapidly in air, forming sodium oxideon its surface.Sodium is extremely reactive. It reacts explosively in water as it releases hydrogen fromthe water with enough heat to ignite the hydrogen. The resulting compound of this reactionis sodium hydroxide (2Na + 2H2O → 2NaOH + H2↑). Due to its extremely electropositivereactivity, there are few uses for the pure metallic form of sodium. Because of its reactivity,hundreds of sodium compounds are found on the Earth’s surface.Guide to the Elements | 51An unusual characteristic of several alkali metals is that a mixture of two or more has alower melting point than the melting point of the separate metals. This is referred to as aeutectic system of metallic alloys. For instance, sodium has a melting point of 97.6°C, andpotassium’s melting point is 63.25°C, but when the two are mixed, the eutectic melting point(turning into a liquid phase) of the combined Na-K system is below zero degrees Celsius(–10°C). If cesium metal (melting point of 38.89°C) is added to the Na and K mixture, themelting point of this eutectic alloy (Na-K-Cs) is the lowest of any eutectic alloy at –78°C.
-
使用
manufacture of sodium Compounds, such as the cyanide, azide, peroxide, etc.; manufacture of tetraethyllead; manufacture of refractory metals; in org syntheses; for photoelectric cells; in sodium lamps; as catalyst for many polymerization reactions. Alloyed with potassium in heat transfer media.
-
定義
sodium: Symbol Na. A soft silveryreactive element belonging to group1 (formerly IA) of the periodic table(see alkali metals); a.n. 11; r.a.m.22.9898; r.d. 0.97; m.p. 97.8°C; b.p.882–889°C. Sodium occurs as thechloride in sea water and in the mineralhalite. It is extracted by electrolysisin a Downs cell. The metal isused as a reducing agent in certainreactions and liquid sodium is also acoolant in nuclear reactors. Chemically,it is highly reactive, oxidizingin air and reacting violently withwater (it is kept under oil). It dissolvesin liquid ammonia to formblue solutions containing solvatedelectrons. Sodium is a major essentialelement required by living organisms.The element was first isolatedby Humphry Davy in 1807.
-
調製方法
Sodium is an essential element needed for all organic life.
Sodium is produced commercially through the electrolysis of
liquid sodium chloride mixed with calcium chloride in a
Downs Cell. Very pure sodium can be isolated by the thermal
decomposition of sodium azide (NaN3).
Sodium, in its metallic form, can be used to refine some
reactive metals, such as zirconium and potassium, from
their compounds and is very important in making esters.
-
一般的な説明
Sodium,Na, melts at 97.8°C and boils at 892°C. It is silver-white in color, is soft and malleable, and oxidizes in air. When exposed to air, a silvery soft metal that becomes grayish white upon. It occurs naturally only in the forms of its salts. Shipped as a solid or molten liquid. Burns violently with explosions that may spatter the material. Sodium is used as a chemical intermediate. and in pharmaceuticals, petroleum refining and metallurgy, electric power cable, Sodium lamps, other chemicals.
-
空気と水の反応
May ignite spontaneously in air. Reacts violently with water to give Sodium hydroxide and hydrogen, which ignites spontaneously [Merck, 11th ed. 1989)]. The ignition temperature of Sodium in air depends on the area of surface exposed: vapor ignites at room temperature; droplets at about 250°F; an agitated pool at 400°F. In the absence of moisture and hydrogen, the reaction is insignificant [Mellor 2 Supp. 2:440 1961].
-
危険性
Sodium as the elemental metal is very dangerous because of its extreme electropositivenature, particularly when it comes in contact with moist air, water, snow, or ice or otheroxidizing agents. It readily gives up electrons to electronegative atoms (nonmetals). In thesereactions, it releases hydrogen gas with enough heat to explosively ignite the hydrogen.
Numerous sodium compounds are hazardous as carcinogens (cancer-causing) and astoxins (poisons) in plants and animals. On the other hand, we benefit greatly from the manycompounds containing the element sodium. We could not live without it.
-
健康ハザード
Sodium reacts with the moisture on skin and other tissues to form highly corrosive
sodium hydroxide. Contact of metallic sodium with the skin, eyes, or mucous
membranes causes severe burns; thermal burns may also occur due to ignition of the
metal and liberated hydrogen.
-
火災危険
Sodium spontaneously ignites when heated above 115 °C in air that has even
modest moisture content, and any sodium vapor generated is even more flammable.
Sodium reacts violently on contact with water and often ignites or explodes the
hydrogen formed. Sodium fires must be extinguished with a class D dry chemical
extinguisher or by the use of sand, ground limestone, dry clay or graphite, or ''Met-
L-X ? " type solids. Water or CO 2 extinguishers must never be used on sodium fires.
-
燃焼性と爆発性
Sodium spontaneously ignites when heated above 115 °C in air that has even
modest moisture content, and any sodium vapor generated is even more flammable.
Sodium reacts violently on contact with water and often ignites or explodes the
hydrogen formed. Sodium fires must be extinguished with a class D dry chemical
extinguisher or by the use of sand, ground limestone, dry clay or graphite, or "Met-
L-X ?" type solids. Water or CO2 extinguishers must never be used on sodium fires.
-
使用用途
ナトリウムは、反応性が高いため、金属を精錬する際の還元剤や触媒として用いることが可能です。
さらに、融点が低く熱伝導率が良いことを利用して、高速増殖炉の冷却剤など、冷却を目的として利用されることもあります。そのほか、が高速道路やトンネルの中で使われていたり、石鹸にナトリウムが含まれていたり、身近なところで広く利用されています。
また、ナトリウムは人体にも欠かすことのできないミネラルで、筋肉や神経を正常に保つ働きを担っている元素です。
-
職業ばく露
A potential danger to those involved in tetra-alkyl lead manufacture using lead-sodium alloy as a reactant; those using sodium as a liquid metal coolant, as a catalyst, or in the manufacture of sodium hydride, borohydride, or peroxide.
-
環境運命予測
Elemental sodium that is released into the environment reacts
almost immediately with water to form sodium hydroxide and
hydrogen gas. Even small quantities of metallic sodium can be
explosive when brought into contact with sources of water; the
formation sodium hydroxide raises the local pH and is
extremely caustic. Sodium cations formed from this reaction
are rapidly absorbed into the surrounding environment to
form a large variety of salts.
-
貯蔵
Safety glasses,
impermeable gloves, and a fire-retardant laboratory coat should be worn at all times
when working with sodium, and the metal should be handled under the surface of an
inert liquid such as mineral oil, xylene, or toluene. Sodium should be used only in
areas free of ignition sources and should be stored under mineral oil in tightly
sealed metal containers under an inert gas such as argon.
-
輸送方法
UN1428 Sodium, Hazard Class: 4.3; Labels: 4.3-Dangerous when wet material. Note: Finely divided sodium is pyrophoric.
-
純化方法
The metal is placed on a coarse grade of sintered-glass filter, melted under vacuum and forced through the filter using argon. The Pyrex apparatus is then re-evacuated and sealed off below the filter, so that the sodium could be distilled at 460o through a side arm and condenser into a receiver bulb which is then sealed off [Gunn & Green J Am Chem Soc 80 4782 1958]. EXPLODES and IGNITES in water.
-
不和合性
A strong reducing agent. A dangerous fire hazard when exposed to heat and moisture. Violent reaction with water, forming NaOH. Violent reaction with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides. halogenated hydrocarbons; phosphorus and phosphorus compounds; sulfur and sulfur compounds; and many other chemicals.
-
廃棄物の処理
Incineration with absorption of oxide fumes.