-
外観
無色澄明の液体
-
性質
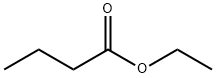
酪酸エチルの示性式は、C3H7COOC2H5です。常温では無色透明な液体として存在し、分子量は116.16、密度は25℃で0.875g/mL、融点は-93.3℃、沸点は120~121℃、引火点は約25℃です。アルコールや油類の有機溶媒にはよく溶けますが、水にはほとんど溶けません。
酪酸エチルには引火性があるため、火気や熱源近くでの使用は避け、使用時の安全管理や保管には十分に配慮する必要があります。酪酸エチルは日本の消防法において、危険物第4類・第2石油類に分類されている物質です。
-
溶解性
エタノールに極めて溶けやすく、水に溶けにくい。
-
解説
酪酸エチル,ラクサンエチル,butanoic acid ethyl ester.C6H12O2(116.16).CH3CH2CH2COOC2H5.硫酸の存在下に酪酸とエタノールとを加熱し,蒸留すると得られる.無色の液体.融点-93.3 ℃,沸点120~121 ℃.d2040.8785.n20D1.3929.有機溶媒に可溶,水に不溶.果実様の香気を有し,甘い風味がある.引火点19 ℃.果実フレーバー,エッセンス香料のほか,種々の溶剤にも用いられる.皮膚に刺激作用があり,蒸気吸入による中毒作用もある.LD50 13000 mg/kg(ラット,経口), > 2000 mg/kg(ウサギ,経皮).
-
用途
食品、飲料(人工ラム酒を含む)の香料剤、石鹸と香水の香り、ラッカーの溶媒。
-
用途
有機合成原料。
-
主な用途/役割
溶剤型接着剤に使用される。
-
説明
Ethyl butyrate, also known as ethyl butanoate, or butyric ether, is an ester with the chemical formula CH3CH2CH2COO.CH2CH3.It is soluble in propylene glycol, paraffin oil, and kerosene.It has a fruity odor, similar to pineapple.
-
化学的特性
Ethyl butyrate is a colorless liquid. Pineapple
odor. The Odor Threshold is 0.015 ppm.
-
天然物の起源
Identified by gas chromatography in olive oil and other vegetable oils. Reported found in apple, banana, citrus
peel oils and juices, cranberry, blueberry, black currants, guava, grapes, papaya, strawberry, onion, leek, cheeses, chicken, beef, beer,
cognac, rum, whiskies, cider, sherry, grape wines, coffee, honey, soybeans, olives, passion fruit, plums, mushroom, mango, fruit
brandies, kiwifruit, mussels and pawpaw.
-
使用
It is commonly used as artificial flavoring resembling orange juice or pineapple in alcoholic beverages (e.g. martinis, daiquiris etc.), as a solvent in perfumery products, and as a plasticizer for cellulose. In addition, ethyl butyrate is often also added to orange juice, as most associate its odor with that of fresh orange juice.
Ethyl butyrate is one of the most common chemicals used in flavors and fragrances. It can be used in a variety of flavors: orange (most common), cherry, pineapple, mango, guava, bubblegum, peach, apricot, fig, and plum. In industrial use, it is also one of the cheapest chemicals, which only adds to its popularity.
-
製造方法
By esterification of n-butyric acid with ethyl alcohol in the presence of Twichell’s reagent or MgCI2; also by heating
n-butyl alcohol and ethanol over CuO + UO3 catalyst at 270°C
-
調製方法
Ethyl Butyrate can be synthesized by reacting ethanol and butyric acid. This is a condensation reaction, meaning water is produced in the reaction as a byproduct.
-
安全性
酪酸エチルは、天然由来成分としてイチゴの果汁、リンゴのみつなどにも存在が確認されている物質です。食品添加物にも使用されることから、低濃度の使用において毒性はありません。
その他の用途においても、大きな危険性は認められていない物質です。ただし、原液などが直接皮膚などに触れた場合は刺激を受ける可能性があり、蒸気状の気体を吸うことによる呼吸器への刺激のおそれがあります。したがって、高濃度で取り扱う場合には注意が必要です。
なお、酪酸エチルの環境に対する有害性は認められていないため、厚生労働省の安全データシートにおいても、水生環境急性有害性、水生環境慢性有害性はいずれも区分外となっています。
-
一般的な説明
A clear colorless liquid with a pineapple-like odor. Flash point 78°F. Less dense than water and insoluble in water. Vapors heavier than air.
-
空気と水の反応
Highly flammable. Insoluble in water.
-
反応プロフィール
Ethyl butyrate is an ester. Esters react with acids to liberate heat along with alcohols and acids. Strong oxidizing acids may cause a vigorous reaction that is sufficiently exothermic to ignite the reaction products. Heat is also generated by the interaction of esters with caustic solutions. Flammable hydrogen is generated by mixing esters with alkali metals and hydrides. May attack some forms of plastics [USCG, 1999].
-
危険性
Irritant to eyes and mucous membranes,
narcotic in high concentration. Flammable, dangerous fire risk.
-
健康ハザード
Inhalation or ingestion causes headache, dizziness, nausea, vomiting, and narcosis. Contact with liquid irritates eyes.
-
火災危険
Behavior in Fire: Vapor is heavier than air and may travel to a source of ignition and flash back. Containers may explode in fire.
-
使用用途
酪酸エチルの主な用途は、食品や飲料に添加される香料です。石鹸や化粧品の香料や等の溶媒、接着剤の溶剤なども用途として挙げられます。まれに、悪臭の目的で用いられることもあります。
酪酸エチルの果実香は、多くの人にバナナやパイナップルを連想させるような匂いです。同じカルボン酸エステルの仲間で酢酸エチルより分子量の小さい酢酸エチルが、リンゴの香りに例えられるのに比べ、やや力強いと表現されることもあります。
-
化学反応性
Reactivity with Water: No reaction; Reactivity with Common Materials: May attack some forms of plastics; Stability During Transport: Stable; Neutralizing Agents for Acids and Caustics: Not pertinent; Polymerization: Not pertinent; Inhibitor of Polymerization: Not pertinent.
-
安全性プロファイル
Wdly toxic by
ingestion. A skin irritant. Flammable liquid
when exposed to heat or flame; can react
vigorously with oxidizing materials. When
heated to decomposition it emits acrid
smoke and irritating fumes. See also
ESTERS.
-
職業ばく露
Ethyl butyrate, and aldehyde, is used
in flavorings, extracts, perfumery, and as a solvent.
-
発がん性
Not listed by ACGIH, California
Proposition 65, IARC, NTP, or OSHA.
-
輸送方法
UN1180; UN1178 Ethyl butyrate, Hazard Class: 3;
Labels: 3-Flammable liquid
-
純化方法
Dry the ester with anhydrous CuSO4 and distil it under dry nitrogen. [Beilstein 2 IV 787.]
-
不和合性
Vapor explosive mixture with air. Ethers
can act as bases. They form salts with strong acids and
addition complexes with Lewis acids. The complex
between diethyl ether and boron trifluoride is an example.
Ethers may react violently with strong oxidizing agents. In
other reactions, which typically involve the breaking of the
carbon-oxygen bond, ethers are relatively inert.
Incompatible with alkaline materials, strong acids, oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause
fires or explosions. Keep away from strong bases and heat
-
廃棄物の処理
Dissolve or mix the material
with a combustible solvent and burn in a chemical incinerator
equipped with an afterburner and scrubber. All federal, state,
and local environmental regulations must be observed