ChemicalBook > CAS DataBase List > β-Estradiol
β-Estradiol
β-Estradiol
- CAS No.50-28-2
- Chemical Name:β-Estradiol
- CBNumber:CB2200244
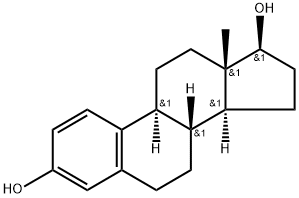
- Molecular Formula:C18H24O2
- Formula Weight:272.39
- MOL File:50-28-2.mol
β-Estradiol Property
- Melting point 178-179 °C(lit.)
- Boiling point 355.44°C (rough estimate)
- alpha D25 +76 to +83° (dioxane)
- Density 1.0708 (rough estimate)
- refractive index 80.4 ° (C=1, Dioxane)
- Flash point 2℃
- storage temp. room temp
- solubility Practically insoluble in water, soluble in acetone, sparingly soluble in ethanol (96 per cent), slightly soluble in methylene chloride.
- form powder
- pka pKa 10.71±0.02(H2O(0.1% p-dioxane) t=25±0.1 I=0.03(KCl))(Approximate)
- color White to off-white
- biological source synthetic (organic)
- Water Solubility Soluble in dimethyl sulfoxide, ethanol , water, phosphate buffer saline, dimethyl formamide, acetone, dioxane and alkali hydroxides. Slightly soluble in vegetable oils.
- Merck 14,3703
- BRN 1914275
- BCS Class 1
- Stability Stable. Incompatible with strong oxidizing agents.
- InChIKey VOXZDWNPVJITMN-ZBRFXRBCSA-N
- CAS DataBase Reference 50-28-2(CAS DataBase Reference)
- EWG's Food Scores 3-6
- NCI Dictionary of Cancer Terms estradiol
- FDA UNII 4TI98Z838E
- NCI Drug Dictionary ESTRING
- ATC code G03CA03,G03CA53
- NIST Chemistry Reference Estra-1,3,5(10)-triene-3,17beta-diol(50-28-2)
- EPA Substance Registry System Estradiol (50-28-2)
- UNSPSC Code 41116107
- NACRES NA.77
Safety
- Hazard Codes :T,Xn,F
- Risk Statements :60-61-45-63-64-40-36-20/21/22-11-48
- Safety Statements :53-22-36/37/39-45-36/37-26-16-36-20
- RIDADR :2811
- WGK Germany :3
- RTECS :KG2975000
- F :8-10
- HazardClass :6.1
- PackingGroup :III
- HS Code :29372390
- Hazardous Substances Data :50-28-2(Hazardous Substances Data)
- Toxicity :LD50 subcutaneous in rat: > 300mg/kg
-
NFPA 704:
1 2 0
-
Symbol(GHS)


- Signal wordDanger
- Hazard statements H351-H360FD-H362-H410
- Precautionary statements P202-P260-P263-P264-P273-P308+P313
β-Estradiol Price
More Price(21)
- Brand: Sigma-Aldrich(India)
- Product number: E8875
- Product name : β-Estradiol
- Purity: ≥98%
- Packaging: 250MG
- Price: ₹4091.85
- Updated: 2022/06/14
- Buy: Buy
- Brand: Sigma-Aldrich(India)
- Product number: E8875
- Product name : β-Estradiol
- Purity: ≥98%
- Packaging: 1G
- Price: ₹8746.6
- Updated: 2022/06/14
- Buy: Buy
- Brand: Sigma-Aldrich(India)
- Product number: E8875
- Product name : β-Estradiol
- Purity: ≥98%
- Packaging: 5G
- Price: ₹34217.83
- Updated: 2022/06/14
- Buy: Buy
- Brand: Sigma-Aldrich(India)
- Product number: E8875
- Product name : β-Estradiol
- Purity: ≥98%
- Packaging: 25G
- Price: ₹136395
- Updated: 2022/06/14
- Buy: Buy
- Brand: Sigma-Aldrich(India)
- Product number: PHR1353
- Product name : Estradiol
- Purity: Pharmaceutical Secondary Standard; Certified Reference Material
- Packaging: 1G
- Price: ₹11593.58
- Updated: 2022/06/14
- Buy: Buy
β-Estradiol Chemical Properties,Usage,Production
-
description
β-Estradiol is an endogenous estrogenic hormone receptor (ER) agonist (Ki values are 0.12 and 0.13 nM for ERα and ERβ respectively). Also high affinity ligand at membrane estrogen GPR30 receptors. β-Estradiol is an activator of PI 3-kinase.
Estradiol (17β-estradiol, β-Estradiol, E2, 17β-Oestradiol) is a human sex hormone and steroid, and the primary female sex hormone. Estradiol upregulates IL-6 expression through the estrogen receptor β (ERβ) pathway. -
Uses
17β-Estradiol is the major estrogen secreted by the premenopausal ovary.This compound is a contaminant of emerging concern (CECs). Drinking water contaminant candidate list 3 (CCL 3) compound as per United States Environmental Protection Agency (EPA), environmental, and food contaminants.
β-Estradiol is used to study cell differentiation and transformations (tumorigenicity). -
Indications and Usage
Estradiol is a white or milky white ordorless crystalline powder. It is soluble in dioxane and acetone, slightly soluble in ethanol, and insoluble in water.
Estradiol is the intermediate between estradiol valerate and estradiol benzoate, and it is a type of estrogen drug. It can be used to treat uterine functional bleeding, primary amenorrhea, menopausal syndrome, and prostate cancer. Estradiol can promote and adjust the normal growth of female sex organs and secondary sex characteristics, promote mammary duct maturation and growth, and aid in posseting. Estradiol can also be used in biochemical research. - Adverse reactions In high dosages, estradiol can inhibit the release of anterior pituitary prolactin, thus decreasing breast milk secretion. However, nausea, vomiting and endometrial hyperplasia-induced bleeding may occur. Patients with liver or kidney failure should use with caution.
- Contradictions Do not use on breasts, vaginal area and vaginal mucosa.
- Description Estradiol, 17-beta- is an odorless white to yellow crystalline substance. Molecular weight = 272.42;Boiling point = (decomposes); Freezing/Meltingpoint = 173 - 179℃. Hazard Identification (based onNFPA-704 M Rating System): Health 2, Flammability 1,Reactivity 0. Insoluble in water.
- Chemical Properties Estradiol, 17-β-is an odorless white to yellow crystalline substance.
- Chemical Properties White or almost white, crystalline powder or colourless crystals.
- Uses 17β-Estradiol is the major estrogen secreted by the premenopausal ovary.This compound is a contaminant of emerging concern (CECs). Drinking water contaminant candidate list 3 (CCL 3) compound as per United States Environmental Protection Agency (EPA), environmental, and food contaminants.
- Uses Estradiol USP (Estrace) is used to treat Breast cancer; prostatic carcinoma.
-
Application
β-Estradiol has been used:
for the in vitro maturation of bovine cumulus-oocyte complexes (COCs)
as a supplement in in vitro maturation medium (IVM), which is used as a control medium
in estrogen-induction assay - Definition ChEBI: The 17beta-isomer of estradiol.
- Acquired resistance Estradiol is the most potent endogenous estrogen, exhibiting high affinity for the ER and high potency when administered parenterally. When administered orally, estradiol is promptly conjugated in the intestine and oxidatively metabolized by the liver, resulting in its low oral bioavailability and therapeutic effectiveness.
-
General Description
Estradiol, estra-1,3,5(10)-triene-3,17β-diol, is the most activeof the natural steroid estrogens. Although its 17β-OHgroup is vulnerable to bacterial and enzymatic oxidation toestrone, it can be temporarily protected as anester at C3 or C17, or permanently protected by adding a17α-alkyl group (e.g., 17α-ethinyl estradiol, the most commonlyused estrogen in oral contraceptives). The increasedoil solubility of the 17β-esters (relative to estradiol) permitsthe esters to remain in oil at the IM injection site for extendedperiods. These derivatives illustrate the principles of steroidmodification. Transdermal estradiolproducts avoid first-pass metabolism, allowing estradiol tobe as effective as oral estrogens for treating menopausalsymptoms. A new transdermal spray, Evamist, was approvedin 2007. Estradiol itself is typically not very effective orallybecause of rapid metabolism, but an oral formulation of micronizedestradiol that allows more rapid absorption of thedrug is available (Estrace). In addition to the oral and transdermalproducts, estradiol is also available in gel, cream, andvaginal ring formulations. The commercially available estradiolesters are the following:
Estradiol 3-acetate, USP (oral; vaginal ring)
Estradiol 17-valerate, USP (IM injection)
Estradiol 17-cypionate, USP (IM injection). - Hazard A carcinogen (OSHA).
- Biological Activity Endogenous estrogen receptor (ER) agonist (K i values are 0.12 and 0.13 nM for ER α and ER β respectively). Also high affinity ligand at membrane estrogen GPR30 receptors.
- Biochem/physiol Actions The major estrogen secreted by the premenopausal ovary. Estrogens direct the development of the female phenotype in embryogenesis and during puberty by regulating gene transcription and, thus, protein synthesis. It also induces the production of gonadotropins which, in turn, induce ovulation. Exposure to estradiol increases breast cancer incidence and proliferation.
- Contact allergens Natural estradiol, used in transdermal systems for hormonal substitution, can induce allergic contact dermatitis, with the risk of systemic contact dermatitis after oral reintroduction.
- Mechanism of action The most potent naturally occurring estrogen in mammals. It is synthesized primarily in the ovary, and also in the testis, adrenal gland and placenta, and to a limited extent by peripheral tissues (e.g., liver, fat, and skeletal muscle) from androstenedione and testosterone. It is responsible for the development of secondary sex characteristics in the female at puberty (i.e., growth and development of the vagina, uterus and fallopian tubes, enlargement of the breasts, and growth and maturation of long bones).
- Safety Profile Confirmed carcinogen with experimental carcinogenic, neoplastigenic, tumorigenic, and teratogenic data. A promoter. Human reproductive effects by ingestion: ferthty effects. Experimental reproductive effects. Human mutation data reported. A steroid hormone much used in medicine. When heated to decomposition it emits acrid smoke and irritating fumes.
-
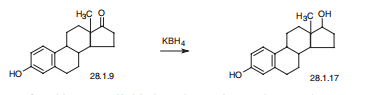
Synthesis
Estradiol, estra-1,3,5(10)-trien-3,17|?-diol (28.1.17), is most easily made by
reducing the keto-group of estrone by various reducing agents, in particular potassium
borohydride.
- Potential Exposure The working environment may be contaminated during sex hormone manufacture, especially during the extraction and purification of natural steroid hormones; grinding of raw materials; handling of powdered products and recrystallization. Airborne particles of sex hormones may be absorbed through the skin, ingested or inhaled. Enteric absorption results in quick inactivation of sex hormones in the liver. The rate of inactivation is decreased for the oral, alkylated steroid hormones (methyl testosterone, anabolic steroids, etc.). Sex hormones may accumulate and reach relatively high levels even if their absorption is intermittent. Consequently, repeated absorption of small amounts may be detrimental to health. Intoxication by sex hormones may occur in almost all the exposed workers if preventive measures are not taken. The effect in the industrial sector is more successful than the agricultural one (chemical caponizing of cockerels by stilbestrol implants and incorporation of estrogens in feed for body weight gain promotion in beef cattle), where measures taken are summary and the number of cases of intoxication is consequently bigger
- First aid If this chemical gets into the eyes, remove anycontact lenses at once and irrigate immediately for at least15 min, occasionally lifting upper and lower lids. Seek medical attention immediately. If this chemical contacts theskin, remove contaminated clothing and wash immediatelywith soap and water. Seek medical attention immediately. Ifthis chemical has been inhaled, remove from exposure,begin rescue breathing (using universal precautions, including resuscitation mask) if breathing has stopped and CPR ifheart action has stopped. Transfer promptly to a medicalfacility. When this chemical has been swallowed, get medical attention. Give large quantities of water and inducevomiting. Do not make an unconscious person vomit.
- storage Room temperature
- Shipping UN3249 Medicine, solid, toxic, n.o.s., Hazard Class: 6.1; Labels: 6.1-Poisonous materials
- Purification Methods 17-Estradiol (previously known as -estradiol) is purified by chromatography on SiO2 (toluene/EtOAc 4:1) and recrystallised from CHCl3/hexane or 80% EtOH. It is stable in air, is insoluble in H2O, and is precipitated by digitonin. The UV has max at 225 and 280 nm. The diacetate [3434-88-6] has m 97-98o and forms leaflets from aqueous EtOH. The 3-benzoate crystallises from aqueous MeOH withm 193o and [] D 25 +58o to 63o (c 1, dioxane). [Meischer & Scholz Helv Chim Acta 20 263, 1237 1937, Biochem J 32 1273 1938, Oppolzer & Roberts Helv Chim Acta 63 1703 1980, Inhoffen & Zühlsdorff Chem Ber 7 4 1914 1941, Beilstein 6 IV 6611.]
- Toxics Screening Level The ITSL for estradiol has been changed from 0.04 μg/m3 to 0.1 μg/m3 based on an annual averaging time.
β-Estradiol Preparation Products And Raw materials
Raw materials
Preparation Products
Global(766)Suppliers
-
Supplier:
CHONGQING CHENCHENG PHARMACEUTICAL CO.,LTD.
- Tel: 15923947264
- Email:lf19823605252@163.com
- Country:China
- ProdList:121
- Advantage:58
-
Supplier:
Wuhan Nutra Biotechnology Co.,Ltd
- Tel: +8617786394783
- Email:nutrabiotech@outlook.com
- Country:China
- ProdList:300
- Advantage:58
-
Supplier:
Wuhan senwayer century chemical Co.,Ltd
- Tel:+undefined-27-86652399<br/>+undefined13627115097
- Email:market02@senwayer.com
- Country:China
- ProdList:923
- Advantage:58
-
Supplier:
Wuhan Quanjinci New Material Co.,Ltd.
- Tel:+86-15271838296;<br/>+8615271838296
- Email:kyra@quanjinci.com
- Country:China
- ProdList:1512
- Advantage:58
-
Supplier:
Shandong Hanjiang Chemical Co., Ltd
- Tel:+86-0533-2066820<br/>+8618369939125
- Email:hanson@sdhanjiang.com
- Country:China
- ProdList:1009
- Advantage:58
-
Supplier:
Shaanxi Xianhe Biotech Co., Ltd
- Tel: +8617709210191
- Email:Jerry@xhobio.com
- Country:China
- ProdList:884
- Advantage:58
-
Supplier:
Hebei Lingding Biotechnology Co., Ltd.
- Tel:+86-18134017493<br/>+86-18134017493
- Email:sunflowerfranklin20@gmail.com
- Country:China
- ProdList:108
- Advantage:58
-
Supplier:
Wuhan Biocar Pharmacy CO.,Ltd.
- Tel:+86-86-13343427080<br/>+8613343427080
- Email:sales@biocarchem.com
- Country:China
- ProdList:492
- Advantage:58
-
Supplier:
Shaanxi Dideu Medichem Co. Ltd
- Tel:+86-29-81148696<br/>+86-15536356810
- Email:1022@dideu.com
- Country:China
- ProdList:3882
- Advantage:58
-
Supplier:
RongNa Biotechnology Co.,Ltd
- Tel:+86-86-13583358881<br/>+8618560316533
- Email:Brad@rongnabiotech.com
- Country:China
- ProdList:3084
- Advantage:58
Related articles
50-28-2, β-EstradiolRelated Search:
- Motolimod Hydrocortisone Estriol Inositol Estradiol Cypionate Acid Red 94 B-ESTRADIOL-3-BENZOATE,BETA-ESTRADIOL BENZOATE,BETA-ESTRADIOL 3-BENZOATE BETA-ESTRADIOL 17-VALERATE ESTRADIOL, 17B-,CIS-ESTRADIOL,B-ESTRADIOL,BETA-ESTRADIOL BETA-ESTRADIOL-17-ENANTHATE 17a-Ethynyl-1,3,5(10)-estratriene-3,17b-diol 3-methyl ether ESTRIOL TRISULFATE TRISODIUM SALT Ethynyl estradiol Quinestrol 2-HYDROXYESTRADIOL BETA-ESTRADIOL 3-METHYL ETHER ESTRIOL TRIACETATE 1,3,5[10]-ESTRATRIENE-3,16ALPHA,17BETA-TRIOL 3-GLUCURONIDE SODIUM SALT Oestradiol
- Steroids
- Hormone
- Estrogen
- Hydroxysteroids
- Estradiol, etc. (Environmental Endocrine Disruptors)
- Environmental Endocrine Disruptors
- Biochemistry
- Analytical Chemistry
- Intracellular receptor
- Steroid and Hormone
- Pharmaceuticals
- Intermediates & Fine Chemicals
- API
- VIVELLE
- Hormone Drugs
- Inhibitors
- powder or crystal
- 雌激素
- 化学原料药
- 杂质对照品
- 抑制剂
- 激素及调节内分泌功能类药
- 醇类
- 高端化学
- 合成有机化合物配体
- 激素原料药
- 医药原料药API
- API医药原料
- 试剂
- 化学试剂
- 临床检测标准物质
- 甾体类原料中间体
- 实验室产品
- 精细化工原料
- 柏锦生物
- 化学原料
- 科研原料
- 通用生化试剂-天然产物
- 通用生化试剂-脂类
- 试剂盒-细胞分析试剂盒
- 化工中间体
- 皮质激素
- 原料药及中间体
- 激素调节类
- 其它原料及中间体
- 化工原料
- 中药对照品
- 医药、农药及染料中间体
- 标准品 -中药标准品
- 其他原料药
- 其它萜类
- 原料
- 医用原料
- 原料药
- 原料药
- 化工原料-原料药
- 对照品
- 生化试剂-其他化学试剂