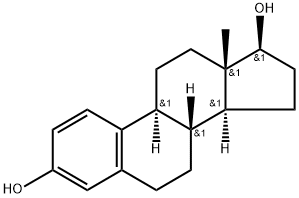
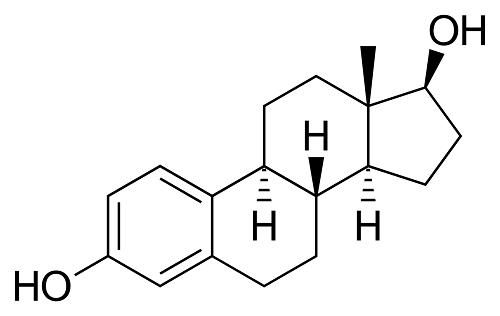
β-Estradiol: Biosynthesis and Mechanism of Action
General Description
β‑estradiol (E2) is a form of estrogen, a female sex hormone produced by the ovaries. Estrogen is necessary for many processes in the body. In vitro, studies indicated that the addition of estradiol to cultures of human lymphocytes decreases CD4+/CD8+ T-cell-subset ratios and enhances immunoglobulin secretion. Studies showed that estrogens can affect immune system cells and may play a role in modulating lymphocyte development and function. Published reports suggest that estrogens may have necessary actions at other steps in the atherogenic process[1].

Uses
β‑estradiol plays a vital role in women’s reproductive health, particularly in developing secondary characteristics such as hips and the menstrual cycle. However, β‑estradiol is the most critical risk factor for breast cancer in most women because estrogen receptors (ERs) are expressed in 75% of breast cancer patients. As a result, β‑estradiol has emerged as a reliable predictor of breast cancer development. WHO (2021) reported that in 2020, the incidence of breast cancer worldwide had reached 11.7% of the 19.2 million new cases of all types of cancer, with 684,996 deaths. Dai et al. classified breast cancer into five subtypes: luminal A, luminal B, HER‑2 positive, triple‑negative, and normal‑like. The luminal subtype, especially luminal A, is most commonly found in breast cancer patients and shows an excellent response to hormone therapy.
Biosynthesis
The discovery of estrogens was soon followed by studies to understand their synthesis and function. It was demonstrated in rats and humans that the enzyme aromatase cytochrome P450 protein converts testosterone to estradiol. The crystal 3D structure of aromatase was, however, only recently solved. The human aromatase gene (P450arom) is encoded by the Cyp19a1 gene and has tissue-specific promoters. Implications of tissue-specific expression and activities of human aromatase are extensively reviewed elsewhere. In females, estradiol (E2) is produced mainly by the developing follicles in the ovary, the corpus luteum and the placenta, while in males, E2 is made in the testes. Small amounts of E2 are also produced by the liver, adrenal glands, and breasts. In postmenopausal women, estrone is the primary circulating estrogen synthesized from dehydroepiandrosterone and secreted by the adrenals. The biosynthesis of E2 from cholesterol and the essential enzymes affecting the critical steps in the pathway are well characterized.
Mechanism of Action
β‐Estradiol, the most potent estrogen hormone in circulation, is involved in a wide variety of vital physiological functions that range from the development and maintenance of reproductive organs to the regulation of cardiovascular, musculoskeletal, immune, and central nervous system homeostasis. Estradiol also contributes to initiating and developing target tissue malignancies[2].
The effects of E2 are mediated by ERα (NR3A1) and ERβ (NR3A2). The dissection of the ER‐mediated E2 signalling in estrogen target tissues largely stems from knock‐out (KO) animal models. Species‐specific differences in tissue distribution withstanding, it appears that ERα predominates. In contrast, ERβ plays a minor role in the uterus, mammary glands, pituitary gland, skeletal muscle, adipose tissue, and bone. Estrogen receptor β, in contrast, is found to be critical in mediating E2 signalling in the ovary, prostate, lung, cardiovascular and central nervous systems. Even within a single tissue, the expression pattern of each subtype is cell type‐specific. For example, ERβ is expressed in the granulosa cells in the ovary, but ERα is more abundant in the theca cells. ERα‐KO and ERβ‐KO mice show different phenotypes reflecting the different ER-subtype distribution patterns. The ERα‐KO female mice are, for example, infertile with a hypertrophic uterus, as well as with anovulatory and hemorrhagic ovaries. In contrast, the ERβ‐KO female mice are subfertile and display reduced ovulation, probably as a result of retardation in granulosa cell differentiation.
Reference
[1] Isti Daruwati. “Method development, validation, and impurity measurement of β-estradiol from radiolabeled [131I]β-estradiol using radio-high-performance liquid chromatography for radioligand of saturation binding assay.” Journal of Advanced Pharmaceutical Technology &Research 14 2 (2023): 105–112.
[2] Pelin Yaşar. “Molecular mechanism of estrogen-estrogen receptor signaling.” Reproductive Medicine and Biology 16 1 (2016): 4–20.
Related articles And Qustion
Lastest Price from β-Estradiol manufacturers
US $0.00-0.00/KG2025-11-24
- CAS:
- 50-28-2
- Min. Order:
- 1KG
- Purity:
- 98
- Supply Ability:
- 10000KGS

US $200.00/g2025-11-24
- CAS:
- 50-28-2
- Min. Order:
- 100g
- Purity:
- 99
- Supply Ability:
- 999