ChemicalBook > CAS DataBase List > 2-HYDROXYESTRADIOL
2-HYDROXYESTRADIOL
2-HYDROXYESTRADIOL
- CAS No.362-05-0
- Chemical Name:2-HYDROXYESTRADIOL
- CBNumber:CB7767256
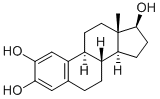
- Molecular Formula:C18H24O3
- Formula Weight:288.38
- MOL File:362-05-0.mol
2-HYDROXYESTRADIOL Property
- Melting point 96-104°C
- Boiling point 370.61°C (rough estimate)
- Density 1.1285 (rough estimate)
- refractive index 1.5000 (estimate)
- storage temp. −20°C
- solubility ≤20mg/ml in ethanol;20mg/ml in DMSO;20mg/ml in dimethyl formamide
- form Solid
- color White to light yellow
- biological source synthetic (organic)
- EWG's Food Scores 1
- FDA UNII AYU2L67YUU
- UNSPSC Code 12352200
- NACRES NA.77
Safety
-
Symbol(GHS)

- Signal wordWarning
- Hazard statements H351
- Precautionary statements P281
2-HYDROXYESTRADIOL Chemical Properties,Usage,Production
- Chemical Properties Pale Yellow Solid
- Uses A metabolite of Estradiol
- Definition ChEBI: A 2-hydroxy steroid that consists of 17beta-estradiol having an additional hydroxy group at position 2.
- Biological Activity 2-hydroxyestradiol is an angiogenesis inhibitor via the hif-1a pathway.hif-1a, a basic helix-loop-helix pas domain containing protein, is reported as the master transcriptional regulator of cellular and developmental response to hypoxia.
- in vitro in vitro evidences suggested that most of the cellular effects of 2-hydroxyestradiol were mediated by 2-methoxyestradiol, a metabolite of 2-hydroxyestradiol as an anti-cancer agent acting as an angiogenesis inhibitor via the hif-1a pathway. inhibition of catechol-o-methyltransferase (comt), the enzyme methylating 2-hydroxyestradiol to 2-methoxyestradiol, blocked the ability of 2-hydroxyestradiol to inhibit growth of vascular smooth muscle cells, cardiac fibroblasts, as well as renal mesangial cells. moreover, 2-hydroxyestradiol could inhibit vascular smooth muscle cell growth in cells obtained from wild-type mice but not in cells cultured from comt knockout mice. in contrast to 2ohe, treatment of vascular smooth muscle cells with 2-methoxyestradiol inhibited serum-induced growth of cells from both wild-type and comtknockout mice [1].
- in vivo animal study showed that after administration of 2-hydroxyestradiol, plasma levels of 2-hydroxyestradiol declined extremely rapidly. concomitant with the disappearance of 2-hydroxyestradiol, 2-methoxyestradiol occurred and then declined. after administration of 2-methoxyestradiol, plasma levels of 2meoe declined with a plasma cl of 50 ml min(-1) kg(-1). we could not detect 2-hydroxyestradiol in plasma from rats receiving 2-methoxyestradiol. thus, the authors conclude that the conversion of 2-hydroxyestradiol to 2-methoxyestradiol was so efficient, and the administration of 2-hydroxyestradiol is bioequivalent to administration of 2-methoxyestradiol itself [1].
- References [1] zacharia, l. c.,piché, c.a.,fielding, r.m., et al. 2-hydroxyestradiol is a prodrug of 2-methoxyestradiol. journal of pharmacology and experimental therapeutics 309(3), 1093-1097 (2004).
2-HYDROXYESTRADIOL Preparation Products And Raw materials
Raw materials
Preparation Products
Global(66)Suppliers
-
Supplier:
Zhengzhou Alfa Chemical Co.,Ltd
- Tel: +8618530059196
- Email:sale04@alfachem.cn
- Country:China
- ProdList:12162
- Advantage:58
-
Supplier:
Hangzhou MolCore BioPharmatech Co.,Ltd.
- Tel:+86-057181025280;<br/>+8617767106207
- Email:sales@molcore.com
- Country:China
- ProdList:49734
- Advantage:58
-
Supplier:
Wuhan Topule Biopharmaceutical Co., Ltd
- Tel: +8618327326525
- Email:masar@topule.com
- Country:China
- ProdList:8467
- Advantage:58
-
Supplier:
Shanghai Acmec Biochemical Technology Co., Ltd.
- Tel:+86-18621343501;<br/>+undefined18621343501
- Email:product@acmec-e.com
- Country:China
- ProdList:33338
- Advantage:58
-
Supplier:
Aladdin Scientific
- Tel:
- Email:tp@aladdinsci.com
- Country:United States
- ProdList:57505
- Advantage:58
-
Supplier:
SHANGHAI KEAN TECHNOLOGY CO., LTD.
- Tel: +8613817748580
- Email:cooperation@kean-chem.com
- Country:China
- ProdList:40066
- Advantage:58
-
Supplier:
DAYANG CHEM (HANGZHOU) CO.,LTD
- Tel: +8617705817739
- Email:info@dycnchem.com
- Country:China
- ProdList:53881
- Advantage:58
- Supplier: J & K SCIENTIFIC LTD.
- Tel: 18210857532
- Email:jkinfo@jkchemical.com
- Country:China
- ProdList:96815
- Advantage:76
- Supplier: Chemsky(shanghai)International Co.,Ltd.
- Tel:021-50135380
- Email:shchemsky@sina.com
- Country:China
- ProdList:32321
- Advantage:50
- Supplier: Shanghai Aladdin Bio-Chem Technology Co.,LTD
- Tel:400-6206333<br/>1316706386
- Email:anhua.mao@aladdin-e.com
- Country:China
- ProdList:25003
- Advantage:65
362-05-0, 2-HYDROXYESTRADIOLRelated Search:
- Estriol 2-Hydroxyestradiol 2-Methyl Ether 4-hydroxyestradiol approx. 90% (hplc),4-HYDROXYESTRADIOL,4-hydroxyestradiol-17bet 16BETA-HYDROXYESTRADIOL,16b-Hydroxyestradiol β-Estradiol 1,3,5(10)-ESTRATRIEN-2,3-DIOL-17-ONE 2-METHYL ETHER 3-ACETATE 17-ETHYLENEKETAL 1,3,5(10)-ESTRATRIEN-2,3,16ALPHA,17BETA-TETROL 2-METHYL ETHER 2-HYDROXYESTRIOL 2-METHOXYESTRADIOL [6,7-3H] 2-METHOXY-17BETA-ESTRADIOL-1,4,16,16,17-D5 2-Hydroxyestradiol-1,4,16,16,17-d5 2-HYDROXY-3,17BETA-O-BIS(METHOXYMETHYL)ESTRADIOL 1,3,5(10)-ESTRATRIEN-2,3-DIOL-17-ONE 3-METHYL ETHER 17-ETHYLENEKETAL 2-HYDROXYESTRADIOL 17a-Ethynyl-2-hydroxyestradiol 3-Methyl Ether 6A-hydroxyestradiol,6ALPHA-HYDROXYESTRADIOL,6α-Hydroxyestradiol 15α-Hydroxyestradiol,15ALPHA-HYDROXYESTRADIOL 2-Hydroxyestradiol 2-Methyl-d3 Ether
- Steroids
- Metabolites & Impurities
- 生化试剂
- 合成
- Steroid Hormones
- Estrogen
- Hormones
- Cytokines, Growth Factors and Hormones
- Cell Biology
- Cell Signaling and Neuroscience
- BioChemical
- 2-HYDROXYESTRADIOL,CYTOCHROME P450 METABOLITE OF ESTRADIOL
- 2-羟基17Β-雌二醇
- DL-2- 雌甾-1,3,5(10)-三烯2,3,17-三醇(13,14,15,16,17,18-13C6,99%)
- 2-羟雌二醇
- 2-羟基雌二醇
- 雌甾-1,3,5(10)-三烯2,3,17-三醇
- 2-羟雌甾二醇
- 1,3,5(10)-雌甾三烯-2,3-17Β-三醇
- 362-05-0
- 2,3,17β-Trihydroxy-1,3,5(10)-Estratriene
- 2-Hydroxyestradiol, Cytochrome P450 metabolite of estradiol
- 1, 3, 5(10)-ESTRATRIEN-2, 3, 17β-TRIOL
- 2-Hydroxy-17β-estradiol
- Estra-1,3,5(10)-triene-2,3,17-triol, (17β)-
- (17b)-Estra-1,3,5(10)-triene-2,3,17-triol
- 1,3,5[10]-ESTRATRIENE-2,3-17BETA-TRIOL
- 1,3,5(10)-ESTRATRIEN-2,3,17-BETA-TRIOL
- 2,3,17BETA-TRIHYDROXY-1,3,5[10]-ESTRATRIENE
- 2-HYDROXYESTRADIOL
- 2-HYDROXY 17-BETA-ESTRADIOL
- Estra-1(10),2,4-triene-2,3,17β-triol
- (17β)-2-Hydroxyestradiol
- 2-OH-E2α
- 2-Hydroxyestradiol-17α
- 1,3,5(10)-Estratriene-2,3-17β-triol, Estra-1,3,5(10)-triene-2,3,17-triol
- estra-1,3,5(10)-triene-2,3,17-beta-triol
- C05301
- 2-Oh-estradiol
- 2-Hydroxyestradiol-17beta
- Estra-1,3,5(10)-triene-2,3,17-triol
- 2-Hydroxy-17-estradiol
- 2,3,17-Trihydroxyestra-1,3,5(10)-triene
- (17)-Estra-1,3,5(10)-triene-2,3,17-triol
- 1,3,5(10)-estratriene-2,3-17β-triol
- NSC 61711
- Estra-1,3,5(10)-triene-2,3,17b-triol
- 2-Hydroxy-17b-estradiol
- 2,3,17b-Trihydroxyestra-1,3,5(10)-triene