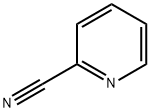
Description
The cyanopyridines are as follows:2-cyano-: A white to tan liquid or solid with an almondodor. Boiling point=about 213℃; Freezing/Meltingpoint=27℃; Flash point=89℃.3-cyano-: A colorless liquid or gray crystalline solid.Molecular weight=104.12; Boiling point=83-84℃;Freezing/Melting point=47-49℃. Hazard Identification(based on NFPA-704 M Rating System): Health 3,Flammability 3, Reactivity 0. Soluble in water.4-cyano-: A beige solid. Freezing/Melting point=75.8℃
Chemical Properties
The cyanopyridines are as follows: 2-cyano-:
A white to tan liquid or solid. Almond odor. Boiling
point=2213℃
; freezing/melting point=27℃
; flash
point=89℃
. 3-cyano-: a colorless liquid or gray crystal-
line solid.
Chemical Properties
white to brown low melting crystalline solid
Uses
It is employed as an important chemical intermediate for rimiterol hydrobromide and used as a bronchodilator. It is also used as intermediates of pharmaceutical and dye and pigment. It acts as a precursor of the respective amidate for protein modification via amidation.
Uses
2-Cyanopyridine is a cyano substituted pyridine. 2-Cyanopyridine is a related compound of nicotine and is a component of tobacco smoke condensate.
Definition
ChEBI: A cyanopyridine carrying the cyano group at position 2.
Potential Exposure
Limits in Air
NIOSH IDLH525 mg/m3
NIOSH REL: (nitriles) 2 ppm, Ceiling Concentration, not
to be exceeded in any 15-minute work period.
First aid
If this chemical gets into the eyes, remove anycontact lenses at once and irrigate immediately for at least15 min, occasionally lifting upper and lower lids. Seek medical attention immediately. If this chemical contacts theskin, remove contaminated clothing and wash immediatelywith soap and water. Seek medical attention immediately. Ifthis chemical has been inhaled, remove from exposure,begin rescue breathing (using universal precautions, including resuscitation mask) if breathing has stopped and CPR ifheart action has stopped. Transfer promptly to a medicalfacility. When this chemical has been swallowed, get medical attention. Give large quantities of water and inducevomiting. Do not make an unconscious person vomit.
storage
Color Code—Blue: Health Hazard/Poison: Storein a secure poison location. Prior to working with thischemical you should be trained on its proper handling andstorage. Store in tightly closed containers in a cool, wellventilated area away from heat or flame and separate fromoxidizing materials
Shipping
UN3276 Nitriles, liquid, toxic, n.o.s., Hazard
Class: 6.1; Labels: 6.1-Poisonous materials, Technical
Name Required, Potential Inhalation Hazard (Special
Provision 5).
Incompatibilities
Oxidizing agents, such as perchlorates,
peroxides, and permanganates. Nitriles may polymerize in
the presence of metals and some metal compounds. They
are incompatible with acids; mixing nitriles with strong
oxidizing acids can lead to extremely violent reactions.
Nitriles are generally incompatible with other oxidizing
agents such as peroxides and epoxides. The combination of
bases and nitriles can produce hydrogen cyanide. Nitriles
are hydrolyzed in both aqueous acid and base to give car-
boxylic acids (or salts of carboxylic acids). These reactions
generate heat. Peroxides convert nitriles to amides. Nitriles
can react vigorously with reducing agents. Acetonitrile and
propionitrile are soluble in water, but nitriles higher than
propionitrile have low aqueous solubility. They are also
insoluble in aqueous acids
.