Description
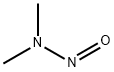
Nitrosamines are chemicals that possess the general structure
R1N(R2)-N=O. These chemicals have been used in the manufacture
of rocket fuel, cosmetics, pesticides, and polymers.
Research studies dating back to the 1950s have demonstrated
that most nitrosamines (>90%) possess some degree of toxicity.
Of particular interest is the nitrosamine N-nitrosodimethylamine
(DMN). This semi-volatile organic
compound is highly toxic and is a suspected human carcinogen.
At higher doses, it has been shown to be a hepatotoxin
that causes liver fibrosis and cancer in several animal species. Its
toxic effects were first reported by British scientists John Barnes
and Peter Magee in 1956 during their screening of chemicals
that were being used as solvents in the dry cleaning industry.
Since then, levels of DMN have been detected in food, drinking
water, soil, and air.
The consumption of contaminated food and water accounts
for the majority of the exposure to DMN. Most nonoccupational
exposure to DMN is a result of chemical reactions
between precursors that form DMN, rather than the industrial
utilization of the chemical itself. For example, after DMN was
discovered in beer in Europe in the 1970s, it was shown that the
direct firedrying of the malt barley used was the DMN source.
Modifications to the drying procedure were able to substantially
reduce the levels of DMN found in beer today. Additionally,
the formation of DMN was attributed to an outbreak
of liver cancers and disorders in livestock that were fed herring
meal in Norway in the 1970s. Subsequent studies showed that
reaction of dimethylamine (naturally occurring in fish) with
nitrosating reagents derived from sodium nitrite (a widely used
preservative) formed the NMD responsible for the liver toxicities.
These studies caused widespread concern over the use of
sodium nitrite in many foods consumed by humans. To
ameliorate the formation of DMN in food caused by sodium
nitrite, manufactures now add antioxidants such as ascorbic
acid (vitamin C), erythorbic acid (an isomer of ascorbic acid),
and a-tocopherol (vitamin E).
DMN has been found in groundwater in many states. Major
sources of DMN in groundwater include rocket fuel production,
and water treatment via chlorination or chloroamination
of wastewater. The removal of DMN from drinking water
usually involves ultraviolet treatment or reverse osmosis.
Chemical Properties
yellow liquid
Chemical Properties
N-Nitrosodimethylamine is a yellow oily liquid.
Faint, characteristic odor.
Physical properties
Yellow, oily liquid with a faint, characteristic odor
Uses
No longer used industrially
or commercially in the US; may occur as a by-product from the manufacture of pesticides,
rubber tires, alkylamines, and dyes
Uses
A highly toxic semi-volatile organic compound and a suspected human carcinogen. It induces liver tumors in rats after chronic exposure to low doses.
Uses
N-Nitrosodimethylamine is a highly toxic semi-volatile organic compound and a suspected human carcinogen. It induces liver tumors in rats after chronic exposure to low doses (1,2). Drinking water contaminant candidate list 3 (CCL 3) compound as per United States Environmental Protection Agency (EPA). Environmental contaminants; Food contaminants.NDMA and NDEA were found as an impurity in generic versions of valsartan, losartan and irbesartan.
Uses
Formerly in the production of rocket fuels; antioxidant; additive for lubricants; softener of copolymers.
Definition
ChEBI: N-nitrosodimethylamine is a nitrosamine. It has a role as a geroprotector and a mutagen.
Production Methods
Dimethylnitrosamine (DMN) is prepared by the addition of
acetic acid and sodium nitrite to dimethylamine. It came
into industrial prominence in the manufacture of 1,1-
dimethylhydrazine, a rocket fuel component, probably in
the 1940s in Germany and in the mid-1950s in the United
States. DMN is no longer used except for research. OSHA
identified DMN as a carcinogen in 1974. In 1976, the
last plant to make DMN was closed. DMN was first considered
by IARC in 1971. The current IARC classification
of DMN is 2A: “The agent is probably carcinogenic
to humans, based upon positive cancer findings in animal
studies.” For this reason, DMN is discussed here as an
example of one of a large group of nitrosamines generally
regarded as carcinogenic. IARC Monographs 1 and
17 and other publications discuss the
toxicology, biochemistry, environmental impact, and carcinogenicity
of many nitrosamines.
General Description
Yellow oily liquid with a faint characteristic odor. Boiling point 151-153°C. Can reasonably be expected to be a carcinogen. Used as an antioxidant, as an additive for lubricants and as a softener of copolymers. An intermediate in 1,1-dimethylhydrazine production.
Air & Water Reactions
Water soluble.
Reactivity Profile
N-NITROSODIMETHYLAMINE is sensitive to exposure to light, especially ultraviolet light. Is stable at room temperature for more than 14 days in aqueous solution at neutral and alkaline pH in the absence of light. Slightly less stable at strongly acid pH at room temperature. Incompatible with strong oxidizing agents. Also incompatible with strong bases. Can be reduced by reducing agents. Incompatible with hydrogen bromide in acetic acid. Also photo chemically reactive.
Health Hazard
N-nitrosodimethylamine
(DMN) is a liver toxin and is carcinogenic in
many species of test animals.
Fire Hazard
When heated to decomposition, N-NITROSODIMETHYLAMINE emits toxic fumes of nitrogen oxides. Avoid exposure to ultraviolet light.
Potential Exposure
Nitrosodimethylamine was formerly
used in the production of rocket fuels. Presently used as
an antioxidant; as an additive for lubricants and as a
softener of copolymers. It is used as an intermediate for
1,1-dimethylhydrazine.
Carcinogenicity
N-Nitrosodimethylamine is reasonably anticipated to be a human carcinogen based on sufficient evidence of carcinogenicity from studies in experimental animals.
Source
After 2 d, N-nitrodimethylamine was identified as a major metabolite of dimethylamine
in an Arkport fine sandy loam (Varna, NY) and sandy soil (Lake George, NY) amended with
sewage and nitrite-N. Mills and Alexander (1976) reported that N-nitrosodimethylamine also
formed in soil, municipal sewage, and lake water supplemented with dimethylamine (ppm) and
nitrite-N (100 ppm). They found that nitrosation occurred under nonenzymatic conditions at
neutral pHs.
Glória et al. (1997) reported N-nitrodimethylamine was detected in 50% of domestic beers at concentrations ranging from 0.05 to 0.50 877 μg/kg with an average value of 0.07 μg/kg. In
imported beers purchased in the U.S., N-nitrodimethylamine was detected in 63% of 78 beers
analyzed at concentrations up to 0.55 μg/kg. The average N-nitrodimethylamine concentration was
0.09 μg/kg.
Environmental Fate
Biological. Two of seven microorganisms, Escherichia coli and Pseudomonas fluorescens, were capable of slowly degrading N-nitrosodimethylamine to dimethylamine (Mallik and Tesfai, 1981).
Photolytic. N-nitrosodimethylamine absorbs UV at 228 nm. An enhanced oxidation process
equipped with UV lamps (195 to 240 nm), mineralized >99.9 % of N-nitrosodimethylamine in
water to concentrations <0.25 μg/L (Smith, 1992). A Teflon bag containing air and
N-nitrosodimethylamine was subjected to sunlight on two different days. On a cloudy day, half of
the N-nitrosodimethylamine was photolyzed in 60 min. On a sunny day, half of the
N-nitrosodimethylamine was photolyzed in 30 min. Photolysis products include nitric oxide,
carbon monoxide, formaldehyde, and an unidentified compound (Hanst et al., 1977).
Chemical/Physical. N-Nitrosodimethylamine will not hydrolyze because it does not contain a
hydrolyzable functional group (Kollig, 1993). Odziemkowski et al. (2000) studied the reduction
mechanism of N-nitrosodimethylamine by granular iron using potentiostatic electrolysis and
differential pulse voltammetry. In the electrochemical experiments, dimethylamine and nitrous
oxide formed. The investigators reported that in an earlier experiment using batch and column
experiments, nitrous oxide, characteristic of electrochemical reduction, was not detected. Rather,
ammonia and dimethylamine were the products identified. The investigators proposed catalytic
hydrogenation was the mechanism for N-nitrosodimethylamine reduction.
Shipping
UN2810 Toxic liquids, organic, n.o.s., Hazard
Class: 6.1; Labels: 6.1-Poisonous materials, Technical
Name Required. PG I.
Purification Methods
Dry the nitrosamine over anhydrous K2CO3 or dissolve it in Et2O, dry it over solid KOH, filter, evaporate Et2O and distil the yellow oily residue through a 30cm fractionating column discarding the first fraction which may contain Me2N. Also dry over CaCl2 and distil it at atmospheric pressure. All operations should be done in an efficient fume cupboard as the vapors are TOXIC and CARCINOGENIC. [Fischer Chem Ber 8 1588 1875, Romberg Recl Trav Chim, Pays-Bas 5 248 1886, Hatt Org Synth Coll Vol II 211 1961, Krebs & Mandt Chem Ber 108 1130 1975.] 2,6-Dimethyl-2,4,6-octatriene see neo-alloocimene below.
Toxicity evaluation
DMN must undergo bioactivation to exert its toxic properties,
and this process is thought to play an important role in
determining tissue- and species-specific toxicity. Bioactivation
of DMN involves oxidation of one of the N-methyl groups to
a primary alcohol by various cytochrome P-450s, followed by
expulsion of a formaldehyde molecule to give an unstable
monomethylnitrosamine product. This then collapses to
produce a highly reactive carbocation, which has been shown
to react with biomolecules such as protein, DNA, and RNA.
Single- and double-strand breaks in DNA and DNA fragmentation
in the form of a ladder have been observed in cells and
tissues.
The bioactivation of DMN is catalyzed by numerous cytochrome
P-450 isoforms, mainly in the liver. Various isoforms
of cytochrome P-450 possess differing affinities for DMN; the
isoforms participating in DMN activation is dependent on
DMN concentration. In humans, for example, P-450IIE1 has
high affinity for DMN and a high turnover number in catalyzing
the methylation and denitrozation of DMN. Its homologue
is present in rats, rabbits, and other animals, and is
inducible by a variety of substances, including acetone,
ethanol, pyrazole, and isoniazid, as well as by physiological
conditions such as fasting and diabetes. In contrast, human
isoform P-450IIBI metabolizes DMN when it is present at high
concentrations. Since animals and humans are rarely exposed
to such high levels of DMN, it is believed that P-450IIE1 is the
isoform responsible for the toxic effects of DMN.
DMN metabolism has been shown to be affected by the
levels of micronutrients. For example, ascorbate deficiency can
result in a net decrease in levels of cytochrome P-450 and
cytochrome b5, and hence lower DMN bioactivation and
toxicity. Interestingly though, excessive intake of ascorbate did
not result in significant enrichment in levels of cytochromes P-
450 and b5.
Incompatibilities
Incompatible with oxidizers (chlorates,
nitrates, peroxides, permanganates, perchlorates, chlorine,
bromine, fluorine, etc.); contact may cause fires or explosions.
Keep away from alkaline materials, strong acids,
especially peracids strong bases. Sensitive to UV light.
Should be stored in dark bottles.
Toxics Screening Level
The initial threshold screening level (ITSL) for N-NITROSODIMETHYLAMINE 0.00007 μg/m3 based on an annual averaging time.
Waste Disposal
Pour over soda ash, neutralize
with HCl, then flush to drain with large volumes of water.
Consult with environmental regulatory agencies for guidance
on acceptable disposal practices. Generators of waste
containing this contaminant (≥100 kg/mo) must conform
with EPA regulations governing storage, transportation,
treatment, and waste disposal.