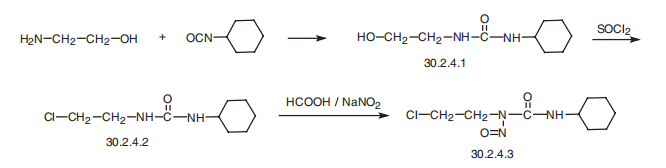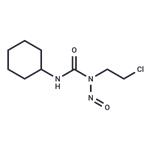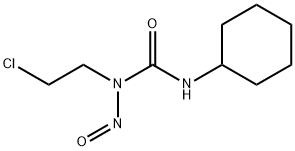
Lomustine
- Product NameLomustine
- CAS13010-47-4
- CBNumberCB5123021
- MFC9H16ClN3O2
- MW233.7
- EINECS235-859-2
- MDL NumberMFCD00012392
- MOL File13010-47-4.mol
- MSDS FileSDS
Chemical Properties
| Melting point | 88-90 |
| Boiling point | 63.6°C (rough estimate) |
| Density | 1.3840 (rough estimate) |
| refractive index | 1.5790 (estimate) |
| storage temp. | 2-8°C |
| solubility | Practically insoluble in water, freely soluble in acetone and in methylene chloride, soluble in ethanol (96 per cent). |
| form | Solid |
| pka | 10.88±0.20(Predicted) |
| color | Light orange to Yellow to Green |
| Merck | 14,5564 |
| InChIKey | GQYIWUVLTXOXAJ-UHFFFAOYSA-N |
| CAS DataBase Reference | 13010-47-4(CAS DataBase Reference) |
| NCI Dictionary of Cancer Terms | CCNU; lomustine |
| FDA UNII | 7BRF0Z81KG |
| NCI Drug Dictionary | lomustine |
| ATC code | L01AD02 |
| IARC | 2A (Vol. 26, Sup 7) 1987 |
| EPA Substance Registry System | 1-(2-Chloroethyl)-3-cyclohexyl-1-nitrosourea (13010-47-4) |
Safety
| Symbol(GHS) |
 
|
|||||||||
| Signal word | Danger | |||||||||
| Hazard statements | H301-H350 | |||||||||
| Precautionary statements | P201-P280-P301+P330+P331+P310-P308+P313 | |||||||||
| Hazard Codes | T | |||||||||
| Risk Statements | 45-25 | |||||||||
| Safety Statements | 53-45 | |||||||||
| RIDADR | 3249 | |||||||||
| WGK Germany | 3 | |||||||||
| RTECS | YS4900000 | |||||||||
| HazardClass | 6.1(a) | |||||||||
| PackingGroup | II | |||||||||
| HS Code | 29299090 | |||||||||
| Hazardous Substances Data | 13010-47-4(Hazardous Substances Data) | |||||||||
| Toxicity | LD50 in male mice (mg/kg): 51 orally; 56 i.p.; 61 s.c. (Thompson, Larson) | |||||||||
| NFPA 704: |
|