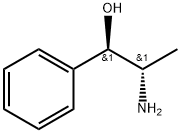
Description
This alkaloid was first isolated by Kanao from the Chinese drug 'Ma-Huang'
(Ephedra sinica Stapf.) and later by Wolfes from E. helvetica C. A. Meyer and E.
distachya Linn. It forms a crystalline mass and has [α]
20
D - 14.56° (EtOH).
Several crystalline salts and derivatives are known: the hydrochloride has m.p.
171-2°C; [α]
20
D- 33.27° (H20); the sulphate dihydrate, m.p. 285-6°C (dry,
dec.); [α]
28
D- 31.99° (H20); the aurichloride, m.p. 188°C; platinichloride, m.p.
221°C (dec.); (-)-hydrogen tartrate, m.p. about 160° after sintering at 130°C;
oxalate, m.p. 245°C (dec.) and the p-nitrobenzoyl derivative, m.p. 175-6°C;
[α]
28
D- 49.58° (CHCI3).
The (+)-form has m.p. 52°C; [α]
27
D + 14.76° (EtOH), yielding a hydro_x0002_chloride, m.p. 171-2°C; [α]
27
D + 33.4° (EtOH); sulphate dihydrate, m.p. 285-
6°C (dry, dec.); [α]
27
D+ 31.51 0 C (H20); aurichloride, m.p. 188°C (dec.) and
platinichloride, m.p. 221.5°C (dec.). The (±)-form crystallizes as colourless
plates from Et20, m.p. 104-5°C and also furnishes a series of crystalline salts
and derivatives.
Chemical Properties
white powder
Uses
A metabolite of Phenmetrazine.
Definition
ChEBI: (-)-norephedrine is an amphetamine that is propylbenzene substituted by a hydroxy group at position 1 and by an amino group at position 2 (the 1R,2S-stereoisomer). It is a plant alkaloid. It has a role as a plant metabolite. It is a member of amphetamines and a phenethylamine alkaloid.
Safety Profile
A human poison by
ingestion. Poison experimentally by
intravenous, subcutaneous, and
intraperitoneal routes. Moderately toxic by
an unspecified route. Human systemic
effects by ingestion: sleep, increased pulse
rate without blood pressure decrease, and
chronic pulmonary edema or congestion,
convulsions, headache, and blood pressure
elevation. Used in production of drugs of
abuse. When heated to decomposition it
emits toxic fumes of NOx.
References
Kanao., Ber., 63,95 (1930)
Wolfes., Arch. Pharrn., 268,87 (1930)
Hey., J. Chern. Soc., 1232 (1930)
Akabori, Momotani., J. Chern. Soc., Japan, 64, 608 (1943)
Hoover, Hass., J. Org. Chern., 12,506 (1947)