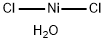Chemical Properties
Green solid
Uses
Nickel(II) chloride hexahydrate may be employed as a catalyst in the high yield synthesis of tetra-substituted pyrrole derivatives. It may be used for the conversion of tetrahydropyranyl (THP) and
tert-butyldimethylsilyl (TBS) ethers to the corresponding hydroxyl compounds.
Uses
Nickel(II) chloride hexahydrate has been used:
- in the preparation of nickel platting solution for galvanostatically coating nickel inside the reduced carbon layer during the synthesis of multiwall carbon nanotube micromotors
- in screening of metals for inflammatory effect on vascular endothelial cells
- as an additive in artificial cerebrospinal fluid (ACSF) to analyse excitatory post synaptic currents (EPSPs) in the hippocampus slices
Uses
Anhydr salt as absorbent for NH3 in gas masks. Hexahydrate for nickel electroplating; manufacture of nickel catalysts.
Definition
ChEBI: A hydrate of nickel chloride containing nickel (in the +2 oxidation state), chloride and water moeities in the ratio 1:2:6.
General Description
Nickel(II) chloride hexahydrate is a nickel salt that can be used as a catalyst. It is cost effective and can be used in a variety of industrial processes. It can also be used in cross-coupling reactions.
Hazard
Confirmed carcinogen.
reaction suitability
reagent type: catalyst
core: nickel
Biochem/physiol Actions
Nickel (Ni) is an essential trace element that plays an important role in the structure and function of DNA, RNA and protein. It has toxic effects on renal, pulmonary and cardiovascular system at higher concentrations. Nickel is implicated in carcinogenesis. Ni2+ ions is a clastogenic agent. Nickel ions (Ni2+) is a non-specific voltage-gated calcium channel blocker.
Safety Profile
Confirmed human
carcinogen. Poison by intraperitoneal and
intravenous routes. Experimental
reproductive effects. Mutation data
reported. Violent reaction with potassium.
When heated to decomposition it emits very
toxic fumes of Cl-. See also NICKEL
COMPOUNDS and CHLORIDES.
Purification Methods
It crystallises from dilute HCl to form the green hexahydrate. At 70o this dehydrates to the tetrahydrate, and at higher temperatures it forms the anhydrous salt. It sublimes in yellow hexagonal scales in a stream of HCl. Store it in a desiccator as it is deliquescent. [Hart & Partington J Chem Soc 104 1943.]