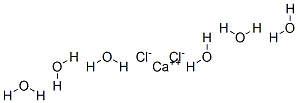
Calcium chloride hexahydrate
- Product NameCalcium chloride hexahydrate
- CAS7774-34-7
- CBNumberCB9218828
- MFCaCl2H12O6
- MW219.08
- EINECS616-496-2
- MDL NumberMFCD00149616
- MOL File7774-34-7.mol
- MSDS FileSDS
Chemical Properties
| Melting point | 30 °C |
| Density | 1.71 g/mL at 25 °C(lit.) |
| refractive index | 1.52 |
| storage temp. | 2-8°C |
| solubility | H2O: 1 M at 20 °C, clear, colorless |
| form | Solid |
| color | Colorless |
| PH | 5.0-7.0 (25℃, 1M in H2O) |
| Odor | Odorless |
| Water Solubility | 5360 g/L (20 ºC) |
| Sensitive | Hygroscopic |
| λmax | λ: 260 nm Amax: 0.018 λ: 280 nm Amax: 0.015 |
| Merck | 14,1659 |
| Stability | Stable. Incompatible with water, zinc, strong acids, methyl vinyl ether, bromine trifluoride, boron oxides. |
| LogP | -1.380 (est) |
| CAS DataBase Reference | 7774-34-7(CAS DataBase Reference) |
| FDA UNII | 1D898P42YW |
| EPA Substance Registry System | Calcium chloride hexahydrate (7774-34-7) |
Safety
| Symbol(GHS) |

|
|||||||||
| Signal word | Warning | |||||||||
| Hazard statements | H319 | |||||||||
| Precautionary statements | P280-P305+P351+P338-P337+P313 | |||||||||
| Hazard Codes | Xi | |||||||||
| Risk Statements | 36 | |||||||||
| Safety Statements | 26-24-22 | |||||||||
| WGK Germany | 1 | |||||||||
| RTECS | EV9830000 | |||||||||
| TSCA | Yes | |||||||||
| HS Code | 28272000 | |||||||||
| NFPA 704: |
|


