Description
Noradrenaline, a catecholamine, is derived from l-tyrosine, an aromatic amino acid present in the body fluids and taken up by noradrenaline-producing cells.
Biological Functions
Most central noradrenergic neurons are located in the nucleus locus ceruleus of the pons and in neurons of the reticular formation. Fibers from these nuclei innervate a large number of cortical, subcortical, and spinomedullary fields. Many functions have been ascribed to the central noradrenergic neurons, including a role in affective disorders, in learning and memory, and in sleep–wake cycle regulation.The mammalian CNS contains both α- and β-adrenoceptors.
Pharmaceutical Applications
Norepinephrine is both a neurotransmitter and a hormone.As a medication, norepinephrine is used to increase and maintain blood pressure in limited, short-term serious health situations.
Pharmacology
The general function of Noradrenalin is to mobilize the brain and body for action. Noradrenalin release is lowest during sleep, rises during wakefulness, and reaches much higher levels during situations of stress or danger, in the so-called fight-or-flight response. In the brain, noradrenalin increases arousal and alertness, promotes vigilance, enhances formation and retrieval of memory, and focuses attention; it also increases restlessness and anxiety. In the rest of the body, noradrenalin increases heart rate and blood pressure, triggers the release of glucose from energy stores, increases blood flow to skeletal muscle, reduces blood flow to the gastrointestinal system, and inhibits voiding of the bladder and gastrointestinal motility.
Clinical Use
Noradrenalin is used to increase and maintain blood pressure in limited, short-term serious health situations.
Side effects
Side effects of norepinephrine as an injection that require medical attention include:
Allergic reactions like skin rash, itching or hives, swelling of your face, lips or tongue.
Difficulty breathing, wheezing.
Irregular heartbeats, palpitations or chest pain.
Pain, redness or irritation at site where injected.
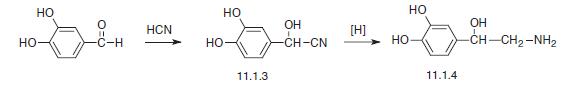
Synthesis
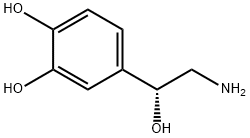
Noradrenalin, L-1-(3,4-dihydroxyphenyl)-2-aminoethanol (11.1.4), is synthesized by two methods starting from 3,4-dihydroxybenzaldehyde. According to the first method, the indicated aldehyde is transformed into the cyanohydrin (11.1.3) by reaction with hydrogen cyanide, which is then reduced into norepinephrine (11.1.5).