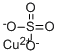Description
Copper sulfate is a greenish-white crystallinesolid; the pentahydrate is blue powder or granules, or ultramarine crystalline solid. Molecular weight =159.60; 249.70(pentahydrate). Boiling point =150℃ (pentahydrate) with25H2O; Melting point = (decomposes) .200℃; decomposes .110℃ (pentahydrate) with 24H2O; copper sulfatedecomposes to CuO at 650℃. Hazard Identification (basedon NFPA-704 M Rating System): Health 2, Flammability 0,Reactivity 0. Highly soluble in water; forms a bright bluesolution.
Chemical Properties
Cupric sulfate, a bluish crystalline powder, also known as hydrocyanite and copper sulfate, vitriol, chalcanthite, and bluestone, is an azure blue material used in the It is used in the leather industry. It is prepared by the reaction of sulfuric acid and copper. It is also obtained as a by-product from copper refineries.
Chemical Properties
Copper sulfate (anhydrous form) is green or gray-white powder, whereas pentahydrate,
the most commonly encountered salt, is bright blue. The anhydrous form occurs as a rare
mineral known as chalcocyanite. Hydrated copper sulfate occurs in nature as chalcanthite.
Copper sulfate is made by the action of sulfuric acid with a variety of copper compounds.
Copper sulfate is used in hair dyes, coloring glass, processing of leather, textiles, and in
pyrotechnics as a green colorant.
Copper sulfate pentahydrate is used as a fungicide and a mixture with lime is called
Bordeux mixture and is used to control fungus on grapes, melons, and other berries, as a
molluscicide for the destruction of slugs and snails, particularly the snail host of the liver
fl uke. Copper sulfate is used in Fehling and Benedict’s solution to test reducing sugars
Chemical Properties
Copper sulfate is a greenish-white crystalline solid; the pentahydrate is Blue powder or granules, or ultramarine crystalline solid.
Uses
Copper Sulfate is a nutrient supplement and processing aid most often used in the pentahydrate form. This form occurs as large, deep blue or ultramarine, triclinic crystals, as blue granules, or as a light blue powder. The ingredient is prepared by the reaction of sulfuric acid with cupric oxide or with copper metal. May be used in infant formula. It is also termed cupric sulfate.
Uses
Used as an antimicrobial and molluscicide.
Uses
Copper sulfate is also known as blue vitriol, this substance was made by the
action of sulfuric acid on elemental copper. The bright-blue
crystals are soluble in water and alcohol. Mixed with ammonia,
copper sulfate was used in liquid filters. The most common
application for copper sulfate was combining it with potassium
bromide for making copper bromide bleach for intensification
and toning. Some photographers used copper sulfate as a
restrainer in ferrous sulfate developers that were used in the
collodion process.
Uses
Copper(II) sulfate may be employed for the following studies:
- As a catalyst for the acetylation of alcohols and phenols under solvent-free conditions.
- To compose the electrolyte for the electrodeposition of Cu-Zn-Sn precursors, required for the preparation of Cu2ZnSnS4 (CZTS) thin films.
- As a Lewis acid catalyst for the dehydration of alcohols.5
Definition
ChEBI: A metal sulfate compound having copper(2+) as the metal ion.
Definition
A compound prepared as the hydrate by the action of dilute sulfuric acid on copper( II) oxide or copper(II) carbonate. On crystallization, blue triclinic crystals of the pentahydrate (blue vitriol, CuSO
4.5H
2O) are formed. Industrially copper(II) sulfate is prepared by passing air through a hot mixture of dilute sulfuric acid and scrap copper. The solution formed is recycled until the concentration of the copper(II) sulfate is sufficient. Copper(II) sulfate is readily soluble in water. The monohydrate (CuSO
4.H
2O) is formed at 100°C and the anhydrous salt at 250°C. Anhydrous copper( II) sulfate is white; it is extremely hygroscopic and turns blue on absorption of water. It decomposes on heating to give copper(II) oxide and sulfur(VI) oxide.
Copper(II) sulfate is used as a wood preservative, a fungicide (in Bordeaux mixture), and in the dyeing and electroplating industries.
General Description
A white or off-white solid. Melting point 200°C with decomposition. Non-combustible.
Air & Water Reactions
Soluble in water.
Reactivity Profile
Anhydrous Cupric sulfate serves as a weak oxidizing agent. Causes hydroxylamine to ignite. Gains water readily. The hydrated salt is vigorously reduced by hydroxylamine [Mellor 8:292(1946-1947)]. Both forms are incompatible with finely powdered metals. Both are incompatible with magnesium, corrode steel and iron, may react with alkalis, phosphates, acetylene gas, hydrazine, or nitromethane, and may react with beta-naphthol, propylene glycol, sulphathiazole and triethanolamine if the pH exceeds 7 . Both act as acidic salts, corrode metals and irritate tissues.
Hazard
Toxic; highly irritant.
Health Hazard
Workers who accidentally ingest copper sulfate experience abdominal pain and cramps,
burning sensation, corrosive effects, nausea, vomiting, loose bowel movement, and a
metallic taste. Exposures to copper sulfate by ingestion or skin absorption cause severe
irritating effects to the eyes and skin The aerosol is irritating to the respiratory tract, and
effects on the blood, kidneys and liver result in hemolytic anemia, kidney impairment,
liver impairment, and shock or collapse. At large doses, accidental intake of copper sulfate
causes renal failure, comatose, and even death. Long-term exposure to copper sulfate may
lead to liver damage, lung diseases, and decreased female fertility.
Health Hazard
INGESTION: copper sulfate may induce severe gastroenteric distress (vomiting, gastroenteric pain, and local corrosion and hemorrhages), prostration, anuria, hematuria, anemia, increase in white blood cells, icterus, coma, respiratory difficulties, and circulatory failure.
reaction suitability
reaction type: click chemistry
Agricultural Uses
Fungicide, Algaecide, Bactericide, Herbicide,
Molluscicide: Copper sulfate is a fungicide used to control bacterial and fungal diseases of fruit, vegetable, nut, and
field crops. These diseases include mildew, leaf spots,
blights, and apple scab. It is used as a protective fungicide
(Bordeaux mixture) for leaf application and seed treatment. It is also used as an algaecide and herbicide, and
to kill slugs and snails in irrigation and municipal water
treatment systems. It has been used to control Dutch elm
disease. It is available as a dust, wettable powder, or liquid
concentrate. Used as a fungicide and algaecide, in veterinary medicine and others. Copper sulfate is also used todetect and to remove trace amounts of water from alcohols
and organic compounds.
Industrial uses
Copper sulfate (CuSO
4·5H
2O) is widely used as an activator for sphalerite, pyrite,
pyrrhotite and other sulfides during processing of base metal ores. During flotation of
some silicate minerals, copper sulfate is used as depressant, e.g. zirconium.
In manufacturing copper sulfate, sulfuric acid and scrap copper metal are used. The
process is based on the oxidation of metal and dissolution with H2SO4 according to the
following reaction:
4Cu + O
2 = 2Cu
2O
Cu
2O + H
2SO
4 = CuSO
4 + H
2O
2Cu
2SO
4 + 2H
2SO
4 + O
2 = 4CuSO
4 + 2H
2O
Usually, in mineral processing applications, copper sulfate is delivered in crystal
form.
Trade name
AGRITOX®; BASICOP®; BCS COPPER
FUNGICIDE®; BSC FLOWABLE®[C]; COPSIN®; CP
BASIC SULFATE®; CUPROFIX®; FUNGI-SPERSE
II[C]; SULTRACOB®; TNCS® 53; TRIANGLE®
Safety Profile
A human poison by
ingestion. An experimental poison by
ingestion, subcutaneous, parenteral,
intravenous, and intraperitoneal routes.
Human systemic effects by ingestion:
gastritis, Qarrhea, nausea or vomiting,
damage to kidney tubules, and hemolysis.
Questionable carcinogen with experimental
tumorigenic data. An experimental
teratogen. Other experimental reproductive
effects. Mutation data reported. Reacts
violently with hydroxylamine, magnesium.
See also COPPER COMPOUNDS and
SULFATES. When heated to
decomposition it emits toxic fumes of SOx
Potential Exposure
Copper sulfate is used as intermediate and wood preservative; also used in production of copper compounds; to detect and to remove trace amounts of water from alcohols and organic compounds; as a fungicide and algicide; in veterinary medicine and others.
First aid
If this chemical gets into the eyes, remove anycontact lenses at once and irrigate immediately for at least15 min, occasionally lifting upper and lower lids. Seek medical attention immediately. If this chemical contacts theskin, remove contaminated clothing and wash immediatelywith soap and water. Seek medical attention immediately. Ifthis chemical has been inhaled, remove from exposure,begin rescue breathing (using universal precautions, including resuscitation mask) if breathing has stopped and CPR ifheart action has stopped. Transfer promptly to a medicalfacility. When this chemical has been swallowed, get medical attention. Give large quantities of water and inducevomiting. Do not make an unconscious person vomit.Note to physician: Empty stomach by lavage with 0.1%solution of potassium ferrocyanide or milk. Liver or kidneyfunction tests may be indicated. May result inmethemoglobinemia
storage
Workers should keep copper sulfate stored in a cool, dry area with suffi cient ventilation. It should be kept away from alkalis, magnesium, ammonia, acetylene, and sodium
hypobromite.
Shipping
UN3288 Toxic solids, inorganic, n.o.s., Hazard Class: 6.1; Labels: 6.1-Poisonous materials, Technical Name Required. UN3077 Environmentally hazardous substances, solid, n.o.s., Hazard class: 9; Labels: 9-Miscellaneous hazardous material, Technical Name Required.
Purification Methods
After adding 0.02g of KOH to a litre of nearly saturated aqueous solution of the sulfate, it is left for two weeks, then the precipitate is filtered on to a fibreglass filter with pore diameter of 5-15 microns. The filtrate is heated to 90o and allowed to evaporate until some CuSO4.5H2O crystallises out. The solution is then filtered hot and cooled rapidly to give crystals which are freed from mother liquor by filtering under suction [Geballe & Giauque J Am Chem Soc 74 3513 1952]. Alternatively crystallise the sulfate from water (0.6mL/g) between 100o and 0o. The pentahydrate is slowly efflorescent, losing 2H2O at 30o, two more H2O are lost at 110o and a white anhydrous powder (dessicant) is obtained on heating above 250o.
Incompatibilities
Aqueous solution is an acid. May form explosive materials on contact with acetylene and nitromethane. Incompatible with strong bases; hydroxylamine, magnesium; zirconium, sodium hypobromite, hydrazine.
Toxics Screening Level
The screening level for Copper(II) sulfate is 10 μg/m3 based on 8 hour averaging.
Waste Disposal
Copper-containing soluble wastes can be concentrated through the use of ion exchange, reverse osmosis, or evaporators to the point where copper can be electrolytically removed and sent to a reclaiming firm. If recovery is not feasible, the copper can be precipitated through the use of caustics and the sludge deposited in a chemical waste landfill Add soda ash to waste CuSO4 solution; let stand 24 hours. Decant and neutralize solution before flushing to sewer. Landfill sludge.
Precautions
During handling and use of copper sulfate, students and occupational workers should
wear safety glasses and should not breathe the material in powder form. Copper sulfate is an environmental pollutant and must be carefully incorporated when used in its varied
applications. Workers should wear protective clothing, goggles, impermeable gloves, and
rubber boots to avoid skin contact