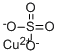ChemicalBook > Articles Catagory List >Inorganic-salts >what-is-the-charge-of-the-metal-ion-in-cuso4
What is the charge of the metal ion in CuSO4?
Feb 18,2024
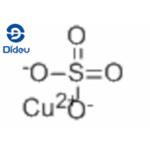
+2 ChargeThe proper name for CuSO4 is copper(II) sulphate - the Roman numerals denote the oxidation state for any atom which has variable oxidation states, such as transition metals.
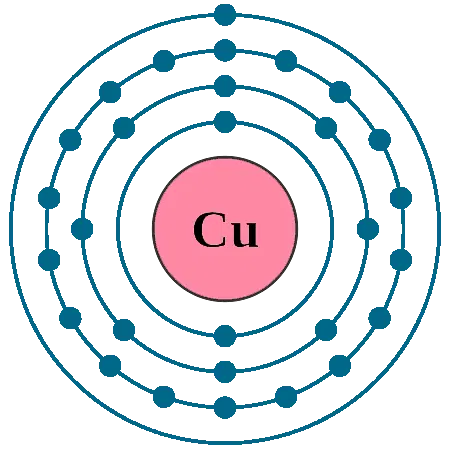
We could start from a knowledge that the sulphate ion has a 2- charge, so the copper ion must have a 2+ charge and hence a +2 oxidation number. We could substitute in the oxidation numbers of the sulphur and oxygen atoms, and apply the rule that:
The Charge On The Compound is 0.
Oxidation Number Of Oxygen is -2.
Oxidation Number Of Sulphur is +6.
So (4 x -2) + 6 = -2 so the copper must be +2.
Oxidation Number Of Cu in Copper Sulphate is +2.
You may like
The Applications of Sodium tungstate dihydrate
Oct 10, 2025
Related articles And Qustion
See also
Lastest Price from Copper(II) sulfate manufacturers
Copper(II) sulfate
US $0.00-0.00/kg2025-12-02
- CAS:
- 7758-98-7
- Min. Order:
- 1kg
- Purity:
- 98%
- Supply Ability:
- 1000kg
Copper Sulfate

US $1200.00-1100.00/ton2025-09-15
- CAS:
- 7758-98-7
- Min. Order:
- 1ton
- Purity:
- 99%
- Supply Ability:
- 1000T/M