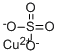Flame color of Copper sulfate and Principle of flame tests
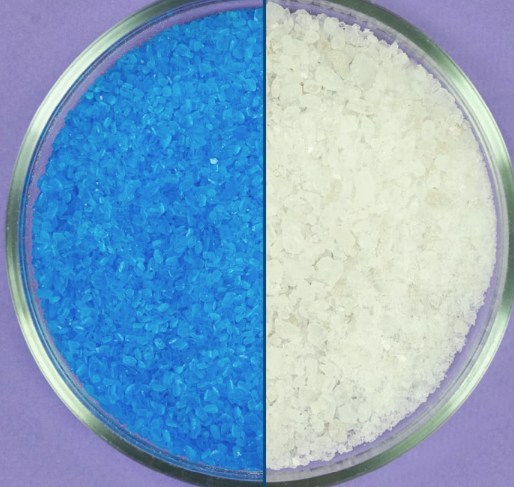
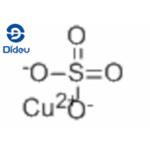
Copper(II) sulfate, also known as copper sulphate, is an inorganic compound with the chemical formula CuSO4. It forms hydrates CuSO4·nH2O, where n can range from 1 to 7. Anhydrous copper sulfate is a light grey powder.

Principle
In flame tests, ions are excited thermally. These excited states then relax to the ground state with emission of a photon. The energy of the excited state(s) and associated emitted photon is characteristic of the element. The nature of the excited and ground states depends only on the element. Ordinarily, there are no bonds to be broken, and molecular orbital theory is not applicable. The emission spectrum observed in flame test is also the basis of flame emission spectroscopy, atomic emission spectroscopy, and flame photometry.
Copper sulfate flame color
When you burned the skewer tip coated with copper sulfate, you should have seen that the flame gained green traces. This is because when the metal copper is burned, it makes green light.
Preparation
Copper sulfate is produced industrially by treating copper metal with hot concentrated sulfuric acid or copper oxides with dilute sulfuric acid. For laboratory use, copper sulfate is usually purchased.
You may like
Related articles And Qustion
See also
Lastest Price from Copper(II) sulfate manufacturers
US $0.00-0.00/kg2025-12-02
- CAS:
- 7758-98-7
- Min. Order:
- 1kg
- Purity:
- 98%
- Supply Ability:
- 1000kg

US $1200.00-1100.00/ton2025-09-15
- CAS:
- 7758-98-7
- Min. Order:
- 1ton
- Purity:
- 99%
- Supply Ability:
- 1000T/M