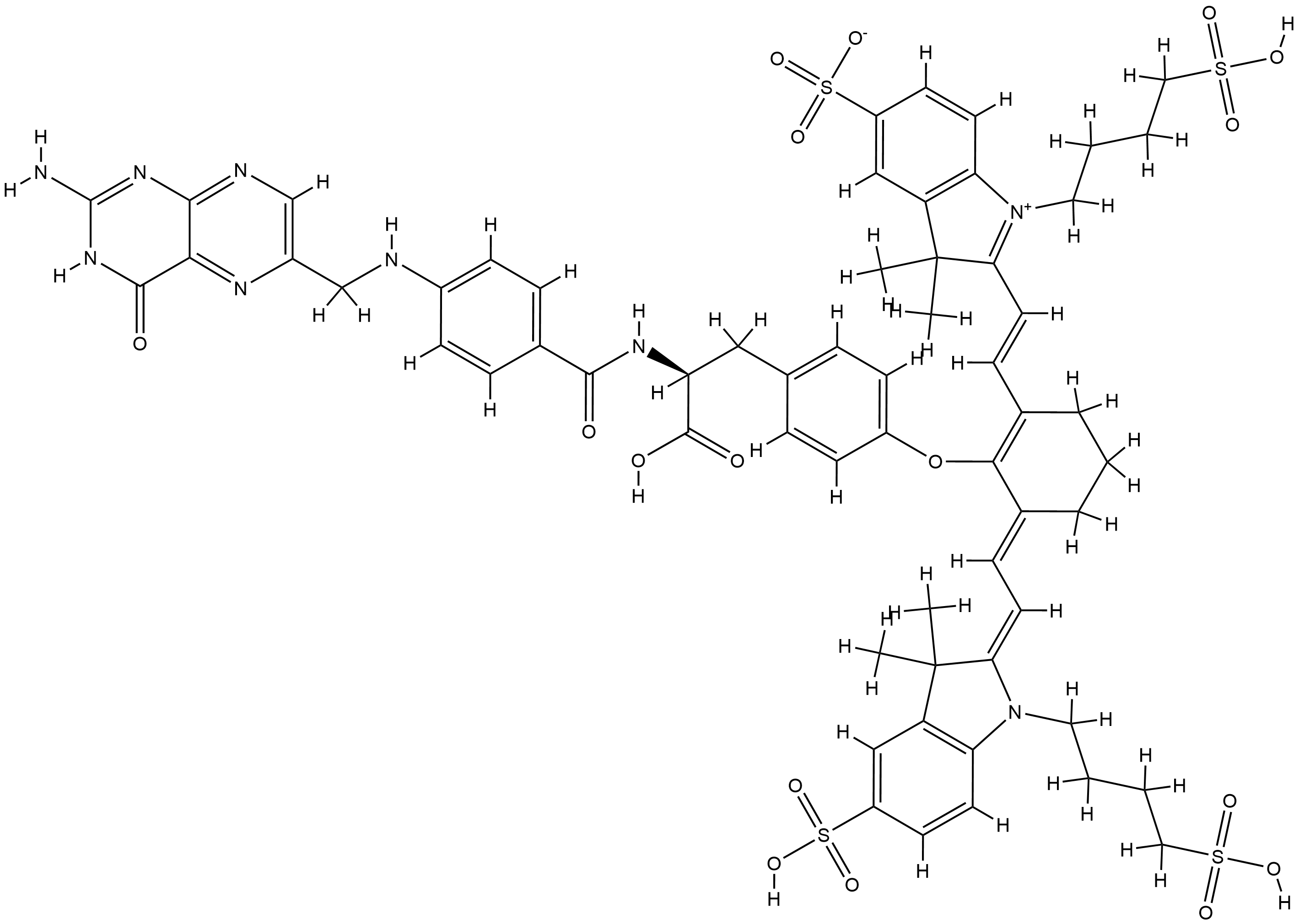
Pafolacianine: Synthesis and Introduction
Synthesis of Pafolacianine
Pafolacianine is synthesised using N10-trifluoroacetylpteroic acid as a raw material by chemical reaction. The specific synthesis steps are as follows:
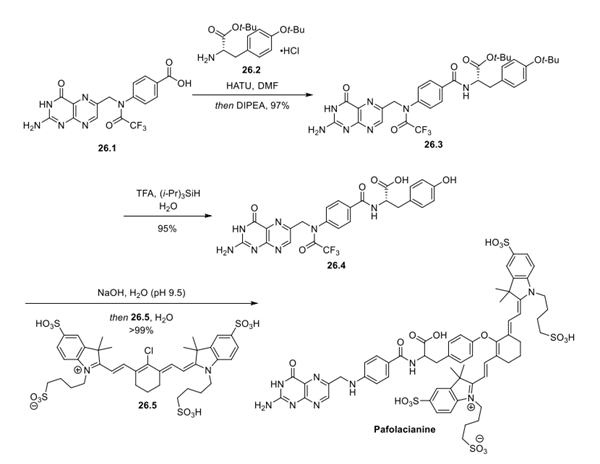
The synthesis begins from N10-trifluoroacetylpteroic acid 26.1, which itself can be generated from pteroic acid via trifluoroacetylation. Amide coupling with t-Bu-protected tyrosine 26.2 produced 26.3 in 97% yield. The t-Bu groups were removed with a mixture of TFA, triisopropylsilane, and water (95:2.5:2.5) to yield 26.4. The trifluoroacetamide was removed with sodium hydroxide in water, and the phenoxide of the tyrosine linker was treated with dye 26.5 to complete the synthesis of Pafolacianine.
Introduction of Pafolacianine
In contrast to traditional small-molecule therapeutics, pafolacianine is an imaging agent. Developed by On Target Laboratories, it has been approved for fluorescence-guided surgery in ovarian cancer. The drug is composed of three parts: a pteroyl group, a tyrosine linker, and a near-infrared dye. The pteroyl group is structurally similar to folic acid and binds to folate receptors that are often overexpressed in ovarian tumors. The tyrosine linker contributes electron density to the dye, enhancing fluorescence. The drug is administered intravenously to patients 2− 3 h before surgery, and a fluorescence imaging system helps guide the surgeon in identifying malignant tissue during the surgery. In clinical studies, it was determined that 29% of the lesions surgically removed using this agent would not have been identified by traditional inspection or palpatation.