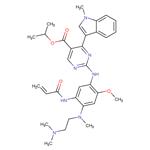
Mobocertinib: Synthesis and Introduction
Synthesis of Mobocertinib
Mobocertinib is synthesised using dichloropyrimidine as a raw material by chemical reaction. The specific synthesis steps are as follows:
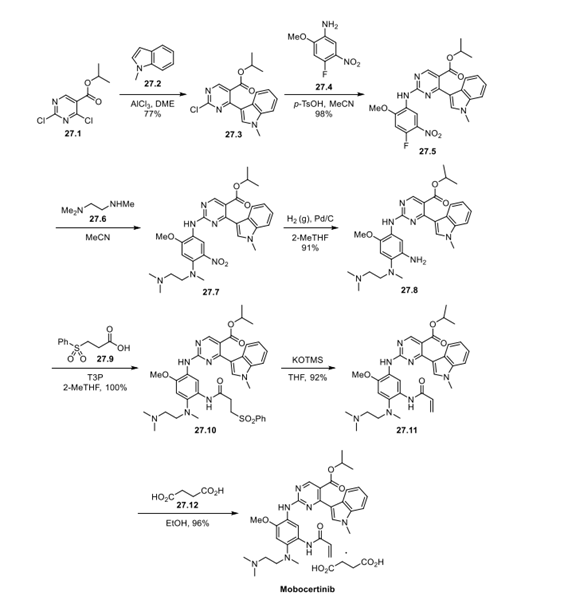
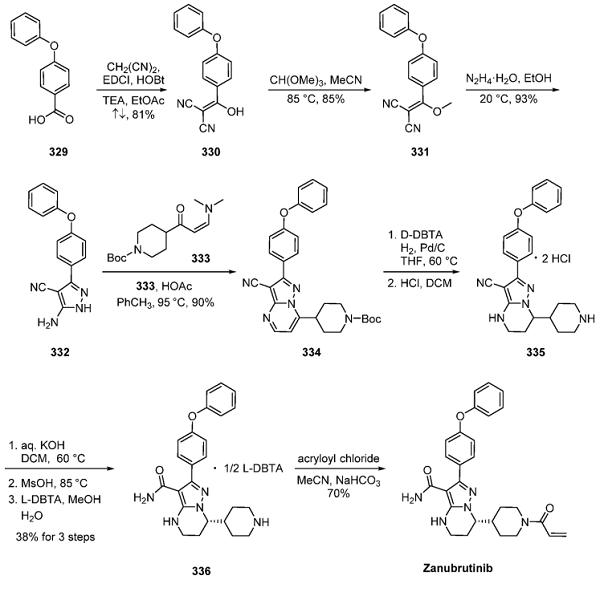
Dichloropyrimidine 27.1 underwent a Friedel−Crafts arylation with indole 27.2, and the second pyrimidinyl chloride was then displaced with aniline 27.4 in an SNAr reaction. A second SNAr reaction of aryl fluoride (27.5) with trimethylethylenediamine (27.6) and reduction of the nitro group yielded aniline 27.8. The acrylamide was introduced in 2 steps: T3P amidation with 27.9 to install the beta-sulfonyl amide (27.10), followed by elimination with potassium trimethylsilanolate to generate 27.11. Mobocertinib (27), administered orally as the succinate salt, was formed by the treatment of 27.11 with succinic acid (27.12) in EtOH.
Introduction of Mobocertinib
Mobocertinib was approved as the first treatment for NSCLC with epidermal growth factor receptor (EGFR) exon 20 insertion mutations in patients whose disease has progressed after platinum-based chemotherapy. The drug, originally discovered by Ariad, which was acquired by Takeda in 2017, is a tyrosine kinase inhibitor (TKI) specifically designed to target the EGFR exon 20 insertion mutation over the wild type. This mutation is present in 2% of all NSCLCs and 4−12% of EGFR-mutated NSCLCs and is difficult to target because its conformation in the ATP binding site is similar to that of the wild type. Mobocertinib acts by forming a covalent attachment with Cys797 of EGFR, and the isopropyl ester moiety can conformationally distinguish the mutated form from the wild type. This drug serves an unmet need, as this mutation typically confers resistance to existing treatments.
You may like
See also
US $0.00-0.00/mk2025-04-20
- CAS:
- 1847461-43-1
- Min. Order:
- 10mk
- Purity:
- 99% HPLC
- Supply Ability:
- 10000