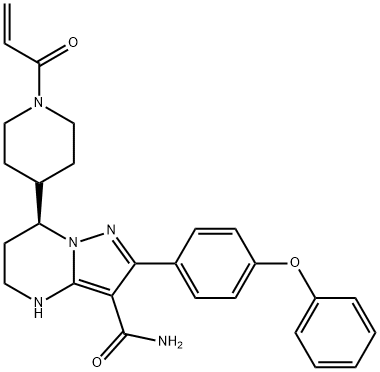
How to synthesize Zanubrutinib?
Description
Zanubrutinib (Brukinsa®), an orally administered Bruton tyrosine kinase (BTK) inhibitor, is being developed by BeiGene to treat B-cell malignancies. Zanubrutinib received accelerated approval in the USA on 14 November 2019 for the treatment of adult patients with mantle cell lymphoma (MCL) who have received at least one prior therapy, based on overall response rate (ORR) seen in phase II and I/II clinical trials[1].
Advantage
Zanubrutinib is a drug that was shown to effectively treat cancer of B cells without causing severe excessive side effects. Patients with certain B-cell malignancies (cancers of white blood cells) benefit from treatment with Bruton tyrosine kinase (BTK) inhibitors. These drugs block the BTK protein and keep cancer from growing and spreading. Patients experience extended survival with ibrutinib, the first-generation BTK inhibitor approved by the US Food and Drug Administration (FDA). However, in kinase inhibition and cell-based assays, zanubrutinib exhibited superior selective BTK inhibition compared with ibrutinib, particularly against EGFR[2]. In addition, one in five patients quit treatment because of harmful side effects. Ibrutinib-related side effects such as increased risk of bleeding, atrial fibrillation (abnormal heart rhythm), and high blood pressure are thought to be caused by ibrutinib blocking other proteins besides the intended target protein BTK. To reduce these side effects, zanubrutinib, a next-generation BTK inhibitor, was designed to block BTK more specifically than ibrutinib. Results of clinical studies on zanubrutinib treatment appear promising in patients with several types of B-cell malignancies, including mantle cell lymphoma (MCL), Waldenström macroglobulinemia (WM), marginal zone lymphoma (MZL), chronic lymphocytic leukemia, and small lymphocytic lymphoma. There is not yet enough clinical data to determine which BTK inhibitor is most effective in treating B-cell malignancies without causing harmful side effects.
Synthesis method
The most likely process synthesis, depicted below, began with benzoic acid 329. Reaction with malononitrile in refluxing ethyl acetate generated vinyl dinitrile 330, which was subsequently methylated using trimethyl orthoformate in warm acetonitrile to generate ether 331. Formation of the corresponding 2- aminopyrazole followed, and this product was immediately reacted with a piperidine-containing keto aldehyde equivalent (vinylogous amide 333), giving rise to zanubrutinib heterocyclic subunit 334. Next, hydrogenative saturation of the fused pyrimidine ring followed by acidic removal of the Boc group provided piperidine 335 as the bis(HCl) salt. The chiral salt resolution next allowed for the isolation of the desired piperidine geometry.
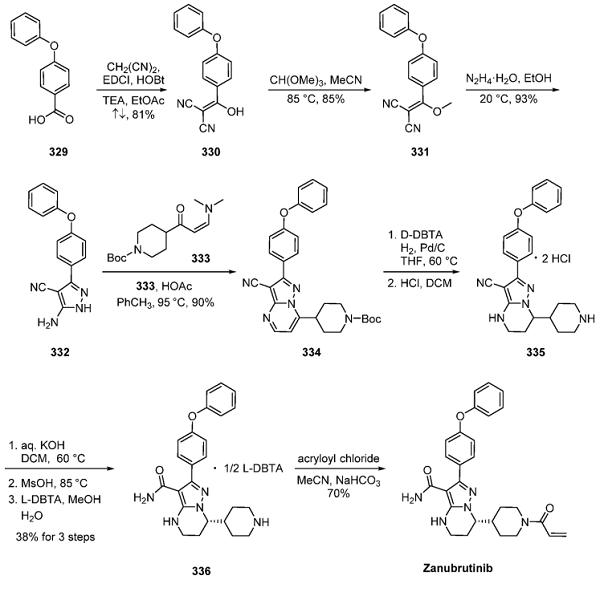
To this effect, exposure of 335 to potassium hydroxide followed by pH adjustment with MsOH and subsequent treatment with L-dibenzoyl tartaric acid (LDBTA) resulted in hydrolysis of the nitrile and isolation of the desired enantiomer as the tartaric acid salt. The reaction of tartrate 336 with acryloyl chloride under Schotten−Baumann conditions furnished zanubrutinib with a 70% yield from 336[3].
References
[1] Syed, Yahiya Y. “Zanubrutinib: First Approval.” Drugs 80 1 (2020): 91–97.
[2] Javier Muñoz. “Zanubrutinib in lymphoproliferative disorders: a comprehensive review.” Therapeutic Advances in Hematology 13 (2022): 20406207221093980.
[3] Andrew C. Flick. “Synthetic Approaches to the New Drugs Approved during 2019.” Journal of Medicinal Chemistry 64 7 (2021): 3604–3657.
You may like
Lastest Price from Zanubrutinib manufacturers

US $10.00/ASSAYS2025-08-29
- CAS:
- 1691249-45-2
- Min. Order:
- 1ASSAYS
- Purity:
- 99%
- Supply Ability:
- 1 ton

US $0.00-0.00/g2025-04-21
- CAS:
- 1691249-45-2
- Min. Order:
- 1g
- Purity:
- 99%
- Supply Ability:
- kg