Carfilzomib: Chemical property, Mechanism of action, and Clinical studies
Introduction
Carfilzomib (also known as PR-171) is an epoxomicin-based proteasome inhibitor with improved pharmaceutical properties. Proteolix Inc. (California, USA) has developed carfilzomib as a second generation proteasome inhibitor to treat multiple myeloma patients. Carfilzomib irreversibly binds to the catalytic site of the proteasome, and inhibits the chymotrypsin-like activity. Unlike bortezomib, carfilzomib has shown minimal cross-reactivity with the other catalytic sites of the 20S CP. Further, carfilzomib shows minimal reactivity with other protease classes. Thus, carfilzomib has a better selectivity than bortezomib for chymotrypsin-like activity of the 26S proteasome in in vitro and in vivo studies. Carfilzomib has also shown better tolerability and dosing flexibility in xenograft models[1].
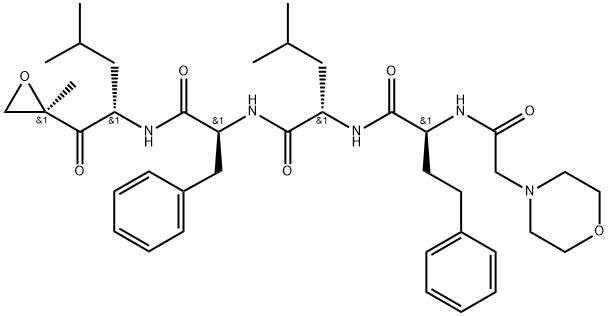
Chemical property
The chemical name for carfilzomib is (2S)-N-((S)-1- ((S)-4-methyl-1-((R)-2-methyloxiran-2-yl)-1-oxopentan-2-ylcarbamoyl)-2-phenylethyl)-2- ((S)-2-(2-morpholinoacetamido)-4-phenylbutanamido)-4-methylpentanamide.
Carfilzomib is a crystalline substance with a molecular weight of 719.9. The molecular formula is C40H57N5O7. Carfilzomib is practically insoluble in water and very slightly soluble in acidic conditions.
Injection
Kyprolis for injection, for intravenous use is a sterile, white to off-white lyophilized powder in a single-dose vial. Each 10 mg vial contains 10 mg of carfilzomib, 500 mg sulfobutylether beta-cyclodextrin, and 9.6 mg anhydrous citric acid and sodium hydroxide for pH adjustment (target pH 3.5). Each 30 mg vial contains 30 mg of carfilzomib, 1500 mg sulfobutylether beta-cyclodextrin, and 28.8 mg anhydrous citric acid and sodium hydroxide for pH adjustment (target pH 3.5). Each 60 mg vial contains 60 mg of carfilzomib, 3000 mg sulfobutylether beta-cyclodextrin, 57.7 mg citric acid, and sodium hydroxide for pH adjustment (target pH 3.5).
Mechanism of action
Carfilzomib is an irreversible inhibitor of the 20S core of the 26S proteasome, an enzyme responsible for degrading cellular proteins. Proteasome inhibition causes accumulation of polyubiquinated proteins, which induces cell cycle arrest and apoptosis. Because carfilzomib is an irreversible inhibitor of this enzyme, it exhibits a more sustained enzyme inhibition compared to bortezomib. Carfilzomib appears to have less off-target activity which may result in a lower incidence of adverse effects such as peripheral neuropathy than bortezomib. Carfilzomib is cell cycle phase-specific and leads to cell cycle arrest at the G2-M phase.
Clinical studies
Pre-clinical studies indicate that carfilzomib is active against models of solid tumors, lymphomas, and myeloma[2]. Carfilzomib inhibits cell proliferation, and induces apoptosis which is associated with activation of JNK (c-Jun N-terminal protein kinase), depolarization of mitochondrial membrane, release of cytochrome C, and activation of both intrinsic and extrinsic caspase pathways in patient-derived multiple myeloma cells as well as neoplastic cells from patients with other haematologic malignancies. The phase I clinical trials of carfilzomib demonstrated that multiple myeloma patients who have relapsed or progressed following a number of therapies (including bortezomib and stem cell transplant) can also achieve durable anti-tumor responses with carfilzomib. Carfilzomib is well tolerated in patients at doses that suppress chymotrypsin-like proteasome activity by > 80% in whole blood. The phase II clinical trials of carfilzomib provided promising results in patients with relapsed or refractory multiple myeloma. Currently, clinical phase III trials are ongoing for carfilzomib in multiple myeloma.
Further, carfilzomib is now under clinical phase I trials for acute myeloid leukemia (AML), acute lymphoblastic leukaemia (ALL), and chronic lymphocytic leukemia (CLL). It is also in phase 1b/II trials in solid tumors. Like bortezomib, carfilzomib may work better in combination with other therapies. In fact, it has been shown to function better in leukemia and lymphoma in combination with HDAC inhibitors in vitro. Carfilzomib has also been shown to interact synergistically with HDAC inhibitors in mantle cell lymphoma cells. Furthermore, carfilzomib acts synergistically with dexamethasone, and has shown an increased level of anti-multiple myeloma activity as compared to bortezomib.Carfilzomib is a proteasome inhibitor.
References
[1] Karel Fostier, Rik Schots, Ann De Becker. “Carfilzomib: a novel treatment in relapsed and refractory multiple myeloma.” OncoTargets and therapy 5 (2012): 237–44.
[2] Sarah Frankland-Searby, Sukesh R. Bhaumik. “The 26S proteasome complex: An attractive target for cancer therapy.” Biochimica et biophysica acta. Reviews on cancer 1825 1 (2012): Pages 64-76.
You may like
Lastest Price from Carfilzomib manufacturers

US $0.00-0.00/kg2025-08-22
- CAS:
- Min. Order:
- 1kg
- Purity:
- 25
- Supply Ability:
- 1

US $0.00-0.00/kg2025-07-01
- CAS:
- 868540-17-4
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 1T