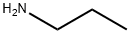Propylamine:Lewis structure,Uses, Preparation and Acute Health Effects
Propylamine is a colorless and volatile liquid. It can be dissolved in various solvents such as water, ethanol, ether, acetone, benzene, and other organic solvents.
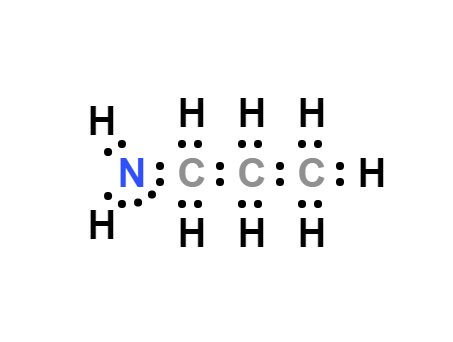
Lewis structure
Properties
Propylamine's specific gravity is 0.7, making it lighter than water. Propylamine has a flammable range of 2% to 10% in air and is flammable. It has a boiling point of 120°F (48°C), flash point of 35°F (37°C), and an ignition temperature of 604°F (317°C). Its vapor density is 2, heavier than air, and can form explosive mixtures when combined with air.
Propylamine can form hydrogen bonds between an N—H bond and an electron pair on a neighboring molecule. Trimethylamine has no N—H bond and therefore cannot form hydrogen bonds. Hydrogen bonding increases the boiling point of propylamine.
Uses
Propylamine is used to make textile resins, drugs, pesticides, and other chemicals.
Preparation
Propylamine hydrochloride can be prepared by reacting 1-propanol with ammonium chloride at high temperature and pressure using a Lewis acid catalyst such as ferric chloride.
Acute Health Effects
The following acute (short-term) health effects may occur immediately or shortly after exposure to Propylamine:
Contact can severely irritate and burn the skin and eyes with possible eye damage. Breathing Propylamine can irritate the nose and throat. Breathing Propylamine can iritate the lungs causing coughing and/or shortness of breath. Higher exposures can cause a build-up of fluid in the lungs (pulmonary edema),a medical emergency, with severe shortness of breath.
You may like
See also
Lastest Price from Propylamine manufacturers
US $1.00/KG2025-04-21
- CAS:
- 107-10-8
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10 mt

US $95.00/kg2024-05-29
- CAS:
- 107-10-8
- Min. Order:
- 1kg
- Purity:
- 99.96%
- Supply Ability:
- 500tons