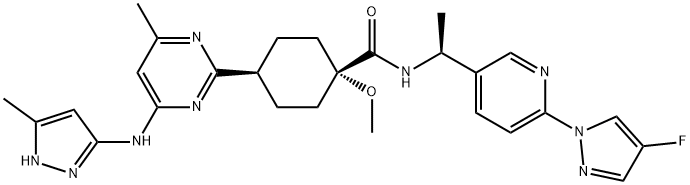
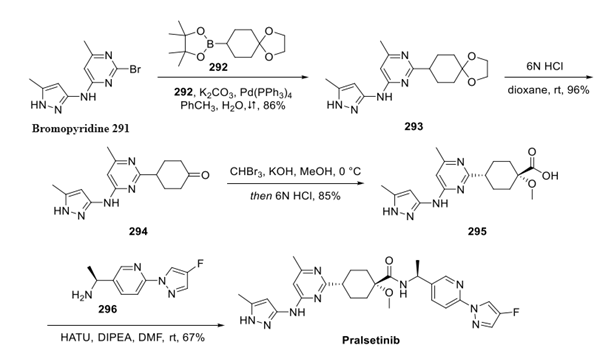
Pralsetinib: Indications, Mechanism of action and Side Effects
Pralsetinib is a kinase inhibitor that targets wild-type RET and kinase-activated RET mutations and fusions. The FDA accelerated the approval of Pralsetinib for non-small cell lung cancer and thyroid cancer on 4 September 2020 and 1 December 2020, respectively.

Indications
Pralsetinib is FDA-approved for treatment:
(1) non-small cell lung cancer (NSCLC)
Patients with NSCLC caused by RET gene rearrangements.
(2) Thyroid cancer
adult patients with metastatic RET fusion-positive NSCLC
adult and pediatric patients ≥12 years of age with advanced or metastatic RET-mutated medullary thyroid cancer requiring systemic therapy
adult and paediatric patients aged ≥12 years with advanced or metastatic RET fusion-positive thyroid cancer requiring systemic therapy and refractory to radioactive iodine (if radioactive iodine is appropriate).
Mechanism of action
Pralsetinib exerts its antitumour effects by specifically inhibiting rearrangements of (RET) tyrosine kinases during transfection, including a number of different oncogenic RET fusions, mutant RET kinase structural domains carrying gatekeeper mutations, and RET kinases with a variety of activating single point mutations.
Rearrangement during transfection (RET) is a transmembrane receptor tyrosine kinase containing extracellular, transmembrane, and intracellular structural domains, the activity of which is required for normal development of the kidney and nervous system.Pralsetinib is a synthetic inhibitor with in vitro IC50 values ranging from 0.3-0.4 nmol/L for WT RET and several mutant forms, including CCDC6-RET. The in vitro IC50 for WT RET and several mutant forms, including CCDC6-RET, ranges from 0.3-0.4 nmol/L, and is 100-fold more selective for RET kinases than 96% of the 371 kinases tested. It is this specific inhibition of RET kinase that is associated with anti-tumour activity and clinical benefit to patients.
Side Effects
The most common clinical adverse reactions to Pralsetinib include diarrhoea, hypertension and fatigue. Other side effects that may occur include: dry mouth, nausea, headache, dizziness, fever, taste disturbance, cough, dyspnoea, rash, decreased appetite and oedema. Serious adverse reactions include: interstitial lung disease/pneumonia, hypertension, hepatotoxicity, bleeding events, risk of impaired wound healing, and tumour lysis syndrome (TLS). Contact your doctor promptly if any of these serious side effects occur.
You may like
Related articles And Qustion
See also
Lastest Price from Pralsetinib manufacturers
US $0.00-0.00/mg2025-04-18
- CAS:
- 2097132-94-8
- Min. Order:
- 10mg
- Purity:
- 98
- Supply Ability:
- 10000000

US $0.00/g2025-01-13
- CAS:
- 2097132-94-8
- Min. Order:
- 1g
- Purity:
- More Than 99%
- Supply Ability:
- 100kg/Month