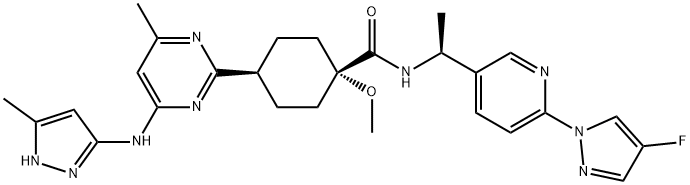
Pralsetinib: Synthesis and Introduction
Synthesis of Pralsetinib
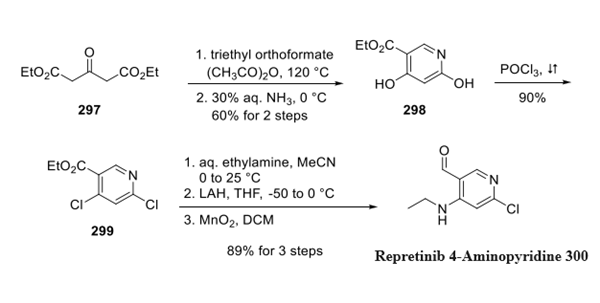
Pralsetinib (BLU-667) is synthesised using bromopyridine as a raw material by chemical reaction. The specific synthesis steps are as follows:
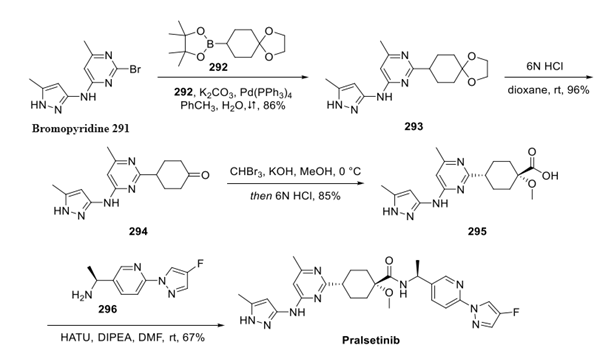
Commercial bromopyridine 291 was subjected to boronate 292 under Suzuki conditions to generate acetonide 293, which was converted to ketone 294 upon acidification. Takenori homologation conditions then established the quaternary carbon within 295, and this was followed by amide coupling with amine 296 to give pralsetinib. The authors indicated that the mild reaction conditions, high product purity, and concise route makes this approach particularly attractive for large scale manufacture.
Introduction of Pralsetinib
Pralsetinib is a tyrosine kinase inhibitor that selectively inhibits "rearranged during transfection" (RET) fusions present in various solid tumors. Blueprint Medicines developed the compound as a treatment for metastatic RET fusion-positive NSCLC. A Phase 1/2 study showed that pralsetinib was safe and efficacious toward RET fusion-positive NSCLC. In September 2020, pralsetinib was approved by the USFDA for lung cancer with RET gene fusions.
You may like
Related articles And Qustion
See also
Lastest Price from Pralsetinib manufacturers
US $0.00-0.00/mg2025-04-18
- CAS:
- 2097132-94-8
- Min. Order:
- 10mg
- Purity:
- 98
- Supply Ability:
- 10000000

US $0.00/g2025-01-13
- CAS:
- 2097132-94-8
- Min. Order:
- 1g
- Purity:
- More Than 99%
- Supply Ability:
- 100kg/Month