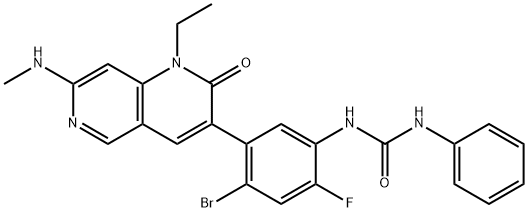
Synthesis of Ripretinib
Synthesis of Ripretinib
Ripretinib was synthesized by construction of a 1,6-naphtholide involving the junction of an α-aryl ester and appropriately functionalized 4-aminopyridine. The specific synthesis steps are as follows:
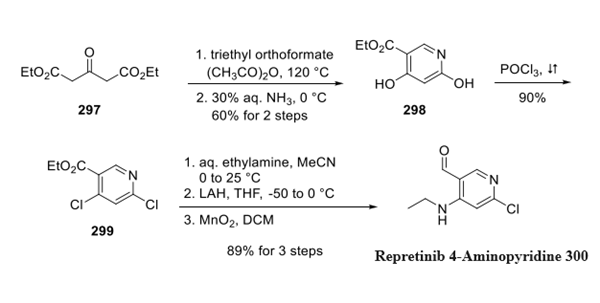
Step 1: Preparation of Repretinib 4-Aminopyridine
The method starts with heating diethyl ester 297 with triethyl orthoformate and acetic anhydride. This was followed by the addition of aqueous ammonia to afford pyridine 298. This compound was then treated with neat phosphorus oxychloride at reflux to provide dichloride 299 in 90% yield. In chilled acetonitrile, the 4-chloro moiety of pyridine 299 wasthen selectively substituted with ethylamine in good yield. This was followed by oxidation state adjustment with lithium aluminum hydride and later MnO2 to furnish 4-aminopyridine 300.
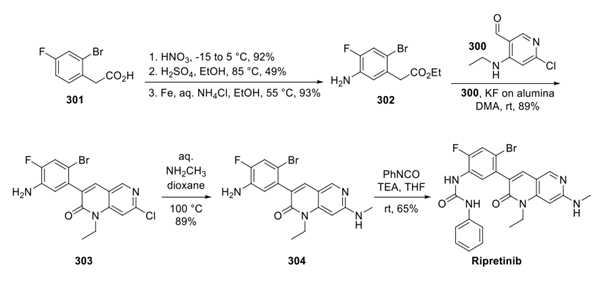
Step 2: Preparation of Ripretinib
Construction of the α-aryl ester building block proceeded in a straightforward manner from acid 301 with a three-step nitration, esterification, and reduction sequence providing ester 302. Next, a modified Friedlander synthesis, whereby ester 302 and intermediate 300 were subjected to KF on alumina in DMA, resulted in a Knoevenagel cyclization to secure the naphthyridone ring system 303. Installation of the methylamine appendage and subsequent urea formation with phenyl isocyanate completed the preparation of ripretinib.
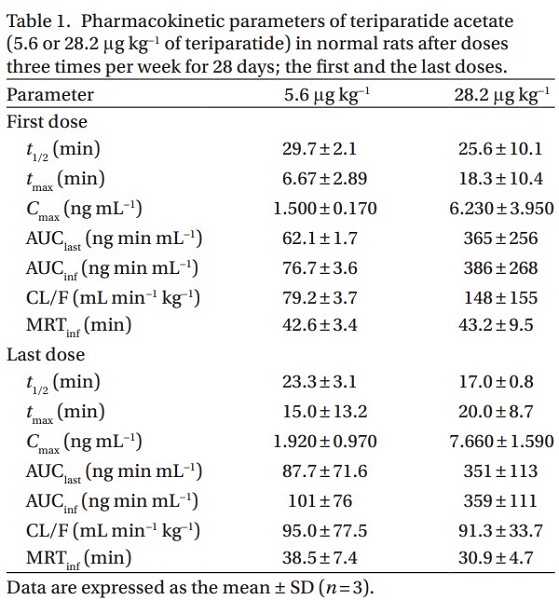
You may like
See also

US $0.00/g2025-01-13
- CAS:
- 1442472-39-0
- Min. Order:
- 1g
- Purity:
- More Than 99%
- Supply Ability:
- 100kg/Month

US $0.00/g2025-01-13
- CAS:
- 1442472-39-0
- Min. Order:
- 1g
- Purity:
- More Than 99%
- Supply Ability:
- 100kg/Month