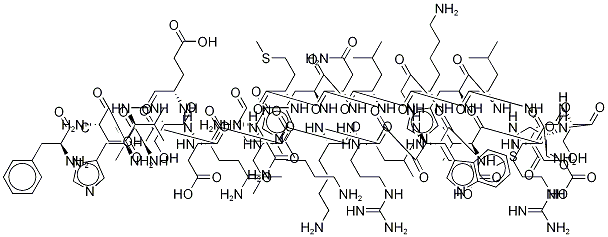
The Pharmacokinetics and uses of Teriparatide acetate
Description
The anabolic drug teriparatide acetate (TA), known as recombinant human parathyroid hormone 1–34, which directly promotes bone formation by generating new osteocytes, has been introduced as a novel therapeutic agent for osteoporosis[1].
Distinct from antiresorptive drug treatment, patients with osteonecrosis of the jaw showed successful clinical outcomes after weekly administration of TA. In addition, adverse outcomes of long-term bisphosphonate treatment, such as bone fracture, were healed after the usage of TA. Better bone mass and mineral density improvements at the lumbar spine and femoral neck were achieved with TA treatment than with bisphosphonate treatment.
Uses
Intravenous administration of hPTH(1-34) increases urinary phosphorus and cAMP via the response of the PTH1 receptor
in the kidneys. Based upon this response, teriparatide acetate was approved for the diagnosis of hypoparathyroidism in Japan in March 1987. Daily injections of low-dose teriparatide increase bone mass and reduce the risk of osteoporotic fracture in postmenopausal women with osteoporosis. On the other hand, weekly injections of teriparatide acetate and synthetic hPTH(1-34) can also prevent bone loss and stimulate new bone formation in patients with primary osteoporosis[2]. Moreover, evidence indicates that weekly teriparatide acetate injections significantly treat osteoporosis and prevent bone fractures.
Pharmacokinetics
In female rats, the Cmax in plasma was reached within 20 minutes of subcutaneous administration of teriparatide acetate (5.6 or 28.2 μg kg−1 of teriparatide). The increases observed in both Cmax and AUC were linear and increased in proportion to the dose (shown below). Additionally, pharmacokinetic parameters measured after three weekly doses over 28 days were not substantially different from those measured after the first dose. Moreover, pharmacokinetic parameters in an ovariectomized rat model of osteoporosis were not different from those of normal animals.
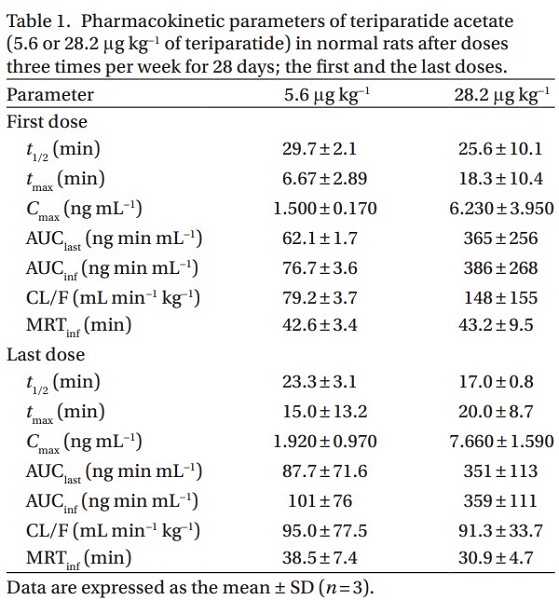
Research has shown that the kidneys can eliminate teriparatide sufficiently, even if the liver cannot metabolize it. Renal failure, but not hepatic failure, affected the clearance of teriparatide acetate, although teriparatide was metabolized in the liver as well as a kidney from the in vitro metabolism study; this finding supposes that in the metabolism of teriparatide, the kidneys can compensate for hepatic failure. However, the liver cannot compensate for renal failure. Therefore, kidneys are thought to be critical for the metabolism of teriparatide.
Teriparatide acetate is rapidly distributed to the circulation following subcutaneous administration and is subsequently distributed primarily to the kidneys and liver, where it is rapidly metabolized into small molecule degradation products. It is thought that the kidneys are not involved in the excretion of teriparatide but rather represent one of the primary organs of distribution and metabolism.
References
[1] Jeeho Sim. “Development of Clinical Weekly-Dose Teriparatide Acetate Encapsulated Dissolving Microneedle Patch for Efficient Treatment of Osteoporosis.” Polymers (2022).
[2] Masashi Serada. “The role of the liver and kidneys in the pharmacokinetics of subcutaneously administered teriparatide acetate in rats.” Xenobiotica 42 4 (2012): 398–407.
You may like
See also
Lastest Price from Teriparatide acetate manufacturers

US $1.00/g2025-09-22
- CAS:
- 52232-67-4
- Min. Order:
- 10g
- Purity:
- 99
- Supply Ability:
- 999

US $1000.00-1000.00/g2025-09-15
- CAS:
- 52232-67-4
- Min. Order:
- 1g
- Purity:
- ≥99% (HPLC); USP43
- Supply Ability:
- 10000000