Physical properties
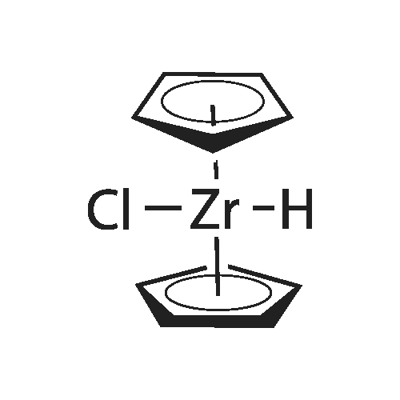
Schwartz Reagent is a white microcrystalline powder. Under the influence of light or heat the color changes to pink, then gradually to dark red.It is sensitive to moisture; it is virtually insoluble in the usual organic solvents such as ethers, consistent with its polymeric character.
Uses
Schwartz Reagent is named after Jeffrey Schwartz, a chemistry professor at Princeton University. This metallocene is used in organic synthesis for various transformations of alkenes and alkynes.
Preparation
Schwartz Reagent can be purchased or readily prepared by reduction of zirconocene dichloride with lithium aluminium hydride:
(C5H5)2ZrCl2 + 1⁄4 LiAlH4 → (C5H5)2ZrHCl + 1⁄4 LiAlCl4
This reaction also affords (C5H5)2ZrH2, which is treated with methylene chloride to give Schwartz Reagent.
Reactions
Schwartz's reagent reacts with alkenes and alkynes via the process called hydrozirconation which formally results in the addition of the Zr-H bond across the C=C or C≡C bond.
Structure and conformation
Schwartz Reagent adopts the usual "clam-shell" structure seen for other Cp2MXn complexes.The dimetallic structure has been confirmed by Microcrystal electron diffraction.The results are consistent with FT-IR spectroscopy, which established that the hydrides are bridging. Solid state NMR spectroscopy also indicates a dimeric structure.