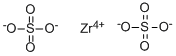Chemical Properties
white crystal(s) [STR93]
Physical properties
Anhydrous sulfate is a microcrystalline hygroscopic solid; density 3.22 g/cm3; decomposes at 410°C; soluble in water.
The tetrahydrate, Zr(SO
4)
2?4H
2O, is a white crystalline solid; orthorhombic crystals; loses three molecules of water between 100 and 150°C; becomes anhydrous at 380°C; soluble in water, 52.5 g/100g solution; the solution deposits a solid on standing; the aqueous solution is strongly acidic, decomposed by bases or on heating.
Uses
Zirconium sulfate [Zr(SO4)2] is an ingredient in lubricants that do not disintegrate at hightemperatures.
It is also used for tanning leather to make it white and as a chemical reagent
and catalyst in chemical laboratories.
Preparation
Zirconium sulfate is prepared by the action of sulfuric acid on zirconium hydroxide: Zr(OH)
4 + 2H
2SO
4 → Zr(SO
4)
2 + 4H
2O
Also, it is prepared by treating zirconyl chloride with hot concentrated sulfuric acid: ZrOCl
2 + 2H
2SO
4 → Zr(SO
4)
2 + H
2O + 2HCl.
General Description
Anhydrous Zirconium sulphate is a colorless microcrystalline solid. Zirconium sulphate is also obtained as a white crystalline tetrahydrate, ZrSO4.4H2O. Both forms are soluble in water and noncombustible. Zirconium sulphate is corrosive to aluminum. Zirconium sulphate is used in chemical analysis, as an additive for lubricants, and for many other uses.
Air & Water Reactions
Water soluble.
Reactivity Profile
Zirconium sulphate is a weak oxidizing agent. Water solutions are acidic and corrosive to aluminum and other metals
Health Hazard
Has only a mild pharmacological action. Inhalation of dust may irritate nose and throat. Contact with eyes or skin causes irritation.
Fire Hazard
Some may burn but none ignite readily. Containers may explode when heated. Some may be transported hot.
Flammability and Explosibility
Non flammable
Safety Profile
Poison by intraperitoneal route. Moderately toxic by
ingestion and subcutaneous routes. A
corrosive. Experimental reproductive
effects. Mutation data reported. When
heated to decomposition it emits toxic
fumes of SOx. See also SULFATES and
ZIRCONIUM COMPOUNDS.