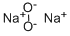
Dinatriumperoxid
Bezeichnung:Dinatriumperoxid
CAS-Nr1313-60-6
Englisch Name:Sodium peroxide
CBNumberCB7853032
SummenformelNa2O2
Molgewicht77.98
MOL-Datei1313-60-6.mol
Synonyma
Dinatriumperoxid
Natriumdioxid
Natriumsuperoxid
Dinatriumperoxid physikalisch-chemischer Eigenschaften
| Schmelzpunkt | 460 °C (dec.)(lit.) |
| Siedepunkt | 657°C |
| chüttdichte | 1138kg/m3 |
| Dichte | 2.8 |
| Dampfdruck | 0.001Pa at 20℃ |
| Flammpunkt | 657°C |
| storage temp. | Store at +15°C to +25°C. |
| Löslichkeit | Soluble in acid. Insoluble in alkali. |
| Aggregatzustand | beads (small) |
| Farbe | Yellow |
| Wichte | 2.805 |
| Geruch (Odor) | Odorless |
| Säure-Base-Indikators(pH-Indikatoren) | 12.8 |
| PH | 12.8 (100g/l, H2O, 20℃) |
| Wasserlöslichkeit | Soluble in water, forming NaOH and H{2}O{2}. Soluble in acid. Insoluble in alkali. |
| Sensitive | Air Sensitive & Hygroscopic |
| Merck | 14,8655 |
| Dielectric constant | 2.7(Ambient) |
| Kennzeichnung gefährlicher | O,C |
| R-Sätze: | 8-35 |
| S-Sätze: | 8-27-39-45 |
| RIDADR | UN 1504 5.1/PG 1 |
| WGK Germany | 1 |
| RTECS-Nr. | WD3450000 |
| F | 3-9-23 |
| TSCA | Yes |
| HS Code | 2815 30 00 |
| HazardClass | 5.1 |
| PackingGroup | I |
| Giftige Stoffe Daten | 1313-60-6(Hazardous Substances Data) |


