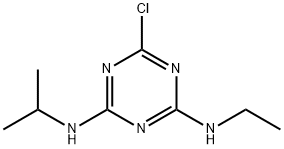
Atrazin
Bezeichnung:Atrazin
CAS-Nr1912-24-9
Englisch Name:Atrazine
CBNumberCB6349448
SummenformelC8H14ClN5
Molgewicht215.68
MOL-Datei1912-24-9.mol
Synonyma
Atrazin
2-Chlor-4-ethylamino-6-isopropylamino-1,3,5-triazin
6-Chlor-N-ethyl-N'-(1-methylethyl)-1,3,5-triazin-2,4-diamin
2-Chlor-4-ethylamino-6-isopropylamino-s-triazin
Atrazin physikalisch-chemischer Eigenschaften
| Schmelzpunkt | 175°C |
| Siedepunkt | 200°C |
| Dichte | 1.187 |
| Dampfdruck | 0Pa at 25℃ |
| Brechungsindex | 1.6110 (estimate) |
| Flammpunkt | 11 °C |
| storage temp. | Keep in dark place,Inert atmosphere,Room temperature |
| Löslichkeit | DMSO: 83.33 mg/mL (386.36 mM) |
| pka | pKa 1.64 (Uncertain) |
| Aggregatzustand | Crystalline |
| Farbe | Crystals |
| Wasserlöslichkeit | Slightly soluble. 0.007 g/100 mL |
| Merck | 14,871 |
| BRN | 612020 |
| Expositionsgrenzwerte | OSHA PEL: TWA 5 mg/m3; ACGIH TLV: TWA 5 mg/m3. |
| Stabilität | Stable. Incompatible with strong oxidizing agents. |
| LogP | 2.59 at 20℃ and pH7.31-7.51 |
| Oberflächenspannung | 57.6mN/m at 30mg/L and 21℃ |
| Dissoziationskonstante | 1.56 at 20℃ |
| CAS Datenbank | 1912-24-9(CAS DataBase Reference) |
| IARC | 3 (Vol. 53, 73) 1999 |
| NIST chemische Informationen | Atrazine(1912-24-9) |
| EPA chemische Informationen | Atrazine (1912-24-9) |
| Kennzeichnung gefährlicher | Xn;N,N,Xn,T,F,Xi |
| R-Sätze: | 43-48/22-50/53-39/23/24/25-23/24/25-11-38-36/37/38-20/21/22-52/53 |
| S-Sätze: | 2-36/37-60-61-45-16-7-36-26 |
| RIDADR | 3077 |
| OEB | B |
| OEL | TWA: 5 mg/m3 |
| WGK Germany | 3 |
| RTECS-Nr. | XY5600000 |
| HazardClass | 9 |
| PackingGroup | III |
| HS Code | 29336990 |
| Giftige Stoffe Daten | 1912-24-9(Hazardous Substances Data) |
| Toxizität | LD50 orally in mice: 1750 mg/kg (Dalgaard-Mikkelsen, Poulsen) |
Gefahreninformationscode (GHS)
-
Bildanzeige (GHS)



-
Alarmwort
Warnung
-
Gefahrenhinweise
H317:Kann allergische Hautreaktionen verursachen.
H373:Kann die Organe schädigen bei längerer oder wiederholter Exposition.
H410:Sehr giftig für Wasserorganismen mit langfristiger Wirkung.
-
Sicherheit
P260:Dampf/Aerosol/Nebel nicht einatmen.
P272:Kontaminierte Arbeitskleidung nicht außerhalb des Arbeitsplatzes tragen.
P273:Freisetzung in die Umwelt vermeiden.
P280:Schutzhandschuhe/Schutzkleidung/Augenschutz tragen.
P302+P352:BEI BERÜHRUNG MIT DER HAUT: Mit viel Wasser/... (Hersteller kann, falls zweckmäßig, ein Reinigungsmittel angeben oder, wenn Wasser eindeutig ungeeignet ist, ein alternatives Mittel empfehlen) waschen.
P314:Bei Unwohlsein ärztlichen Rat einholen / ärztliche Hilfe hinzuziehen.
Atrazine Chemische Eigenschaften,Einsatz,Produktion Methoden
-
ERSCHEINUNGSBILD
FARBLOSE KRISTALLE. -
CHEMISCHE GEFAHREN
Zersetzung beim Erhitzen unter Bildung giftiger Rauche mit Chlorwasserstoff, Stickstoffoxiden. -
ARBEITSPLATZGRENZWERTE
TLV: 5 mg/m?(als TWA); Krebskategorie A4 (nicht klassifizierbar als krebserzeugend für den Menschen); (ACGIH 2005).
MAK: 2 mg/m?(Einatembare Fraktion); Spitzenbegrenzung: überschreitungsfaktor II(8); (DFG 2005).
-
AUFNAHMEWEGE
Aufnahme in den Körper durch Inhalation und durch Verschlucken. -
INHALATIONSGEFAHREN
Beim Verdampfen bei 20 °C tritt eine gesundheitsschädliche Kontamination der Luft nicht oder nur sehr langsam ein, viel schneller jedoch beim Versprühen oder Dispergieren. -
WIRKUNGEN BEI KURZZEITEXPOSITION
WIRKUNGEN BEI KURZZEITEXPOSITION:
Die Substanz reizt die Augen. -
WIRKUNGEN NACH WIEDERHOLTER ODER LANGZEITEXPOSITION
Wiederholter oder andauernder Hautkontakt kann zu Hautsensibilisierung führen. Möglich sind Auswirkungen auf Leber und Nieren. Möglicherweise krebserzeugend für den Menschen. -
LECKAGE
NICHT in die Kanalisation spülen. Verschüttetes Material in Behältern sammeln; falls erforderlich durch Anfeuchten Staubentwicklung verhindern. Reste sorgfältig sammeln. An sicheren Ort bringen. Persönliche Schutzausrüstung: Atemschutzgerät, P2-Filter für schädliche Partikel. -
R-Sätze Betriebsanweisung:
R43:Sensibilisierung durch Hautkontakt möglich.
R48/22:Gesundheitsschädlich: Gefahr ernster Gesundheitsschäden bei längerer Exposition durch Verschlucken.
R50/53:Sehr giftig für Wasserorganismen, kann in Gewässern längerfristig schädliche Wirkungen haben.
R39/23/24/25:Giftig: ernste Gefahr irreversiblen Schadens durch Einatmen, Berührung mit der Haut und durch Verschlucken.
R23/24/25:Giftig beim Einatmen, Verschlucken und Berührung mit der Haut.
R11:Leichtentzündlich.
R38:Reizt die Haut.
R36/37/38:Reizt die Augen, die Atmungsorgane und die Haut.
R20/21/22:Gesundheitsschädlich beim Einatmen,Verschlucken und Berührung mit der Haut. -
S-Sätze Betriebsanweisung:
S2:Darf nicht in die Hände von Kindern gelangen.
S36/37:Bei der Arbeit geeignete Schutzhandschuhe und Schutzkleidung tragen.
S60:Dieses Produkt und sein Behälter sind als gefährlicher Abfall zu entsorgen.
S61:Freisetzung in die Umwelt vermeiden. Besondere Anweisungen einholen/Sicherheitsdatenblatt zu Rate ziehen.
S45:Bei Unfall oder Unwohlsein sofort Arzt zuziehen (wenn möglich, dieses Etikett vorzeigen).
S16:Von Zündquellen fernhalten - Nicht rauchen.
S7:Behälter dicht geschlossen halten.
S36:DE: Bei der Arbeit geeignete Schutzkleidung tragen.
S26:Bei Berührung mit den Augen sofort gründlich mit Wasser abspülen und Arzt konsultieren. -
Beschreibung
A major effort in evaluating the toxicity of the triazines is the cumulative risk assessment (CRA) conducted by US Environmental Protection Agency (EPA) as part of the tolerance reassessment process under the Food Quality Protection Act (FQPA) of 1996. The CRA (released 2006) was conducted for triazines as a common mechanism group (CMG), determined to have a ‘common mechanism of toxicity’ in acting the same way in the body, that is, the same toxic effect occurs in the same organ or tissue by essentially the same sequence of major biochemical events. EPA determined that atrazine, simazine, propazine, and the metabolites desethyl-s-atrazine (DEA), desisopropyl-s-atrazine (DIA), and diaminochlorotriazine (DACT) are considered as a CMG due to their ability to cause neuroendocrine- and endocrine-related developmental, reproductive, and carcinogenic effects. Other triazines, such as ametryn, prometryn, prometon, metsulfuron methyl, trisulfuron, chlorsulfuron, and DPX-M6316, were excluded because these triazines do not share the toxicity profile of the CMG triazines. Hydroxyatrazine was excluded based on the lack of mammary tumor induction and no compelling evidence of neuroendocrine-related toxicity. Propazine was excluded from the cumulative assessment group (CAG) because exposures to propazine are not anticipated via any of the relevant exposure pathways. Therefore, the cumulative assessment included only atrazine, simazine, DEA, DIA, and DACT, referred to as ‘triazine residues.’
For the triazines, the major toxicity of concern involves the neuroendocrine system with the key toxicity mechanism being luteinizing hormone (LH)-dependent effects. The changes in circulating endocrine hormones regardless of rat strain is the basis for assuming commonality of mechanism, which were noted in the same range of doses for these triazines.
The relevance of the induction of mammary tumors in female Sprague–Dawley (SD) rats to humans continues to be a subject of discussion and research on the endocrine effects of triazines. Another consideration is whether the chemicals’ effects on endocrine responses have an impact on reproduction, development, and the brain related or unrelated to carcinogenesis. Significant research into the mechanism of mammary tumor formation was conducted in which the effects of atrazine, simazine, and other triazines were studied on estrus cycle, estrogen-mediated responses, estrogen receptor binding, and hormonal induction and metabolism in several species, but mostly in the rat. Both the in vivo and in vitro data suggest that atrazine and simazine disrupt ovarian cycling and induce mammary tumors in female SD rates, and alteration of the estrous state is directly associated with the incidence of mammary tumors.
Atrazine and its metabolites appear to affect reproductive function of the male as well as the female reproductive and development parameters. However, they have not been tested with exposure at all critical periods of development in the young, evaluated in standard guideline neurotoxicity assay, and the earlier reproductive toxicity studies did not include sensitive measures of endocrine disruption that are now included. Additional studies have been published since the CRA in 2006. The US EPA FIFRA panel reevaluated the database and reaffirmed the conclusion on the toxicity of the triazines and the mammary tumor determination in 2010. -
Chemische Eigenschaften
Atrazine is a colorless, crystalline solid. Although atrazine is very stable, it is only slightly soluble in water, but soluble in N-pentane, chloroform, dimethyl sulfoxide, ethyl acetate, diethyl ether, and methanol. Atrazine is a broad-spectrum triazine herbicide and is used as a selective herbicide for weed control in corn and asparagus, in the culture of sugarcane and pineapple. Additionally, it is used as a total herbicide on roads and public places as well as on uncultivated ground in combination with amitrol, bromacil, dalapon, and growth promoters. Atrazine inhibits photosynthesis and other metabolic processes in plants. There are no natural sources of atrazine. It is produced from cyanuric acid chloride with ethylamine and isopropylamine. The reaction takes place successively in tetrachloromethane. All atrazine produced is released into the environment. The formulations include granules, water dispersible granules, liquid, suspension concentrate, wettable powder, and a combination with many other herbicides. Atrazine is compatible with various insecticides and fungicides.
Atrazine was banned in the European Union (EU) in 2004 because of its persistent groundwater contamination. In the United States, however, atrazine is one of the most widely used herbicides, with 76 million pounds of it applied each year. It is probably the most commonly used herbicide in the world. -
Verwenden
Preemergence and postemergence herbicide for control of some annual grasses and broad-leaved weeds in corn, fallow land, rangeland, sorghum, non-cropland, certain trop ical plantations, evergreen nurseries, fruit crops and lawns. -
Verwenden
Chlorotriazine herbicides have the characteristic triazine (three-nitrogen) aromatic ring, with one chlorine substituent. Chloro-s-triazines may have substitution at the R1 (2 position) by chlorine, thiomethyl, or methoxy. The more extensively studied ones include atrazine (6-chloro-N-ethyl-N0-isopropyl- 1,3,5-triazine-2,4-diamine), simazine (2-chloro-4,6-bis (ethylamino)-s-triazine), propazine (2-chloro-4,6-bis (isopropylamino)-s-triazine), and terbuthylazine (2-(tertbutylamino)- 4-chloro-6-(ethylamino)-s-triazine).
Atrazine and simazine are selective pre- and postemergence herbicides used on crops for control of broad leaf and grassy weeds and in rights-of-way maintenance. Atrazine, first marketed in 1957, is widely used on cauliflower, corn, sorghum, and sugarcane, and in noncropped areas such as wheat fallow. Simazine, introduced in 1956, is used on corn, almonds, grapes, and oranges. Major triazine use occurs in the midwestern cornbelt region of the United States. -
Verwenden
Atrazine is widely used as a selective herbicide to control broadleaf and grassy weeds in corn, sorghum, rangeland, sugarcane, orchards, pineapple, and turf grass sod. It is also used for selective weed control in conifer restoration and Christmas tree plantations. It is also used as a nonselective herbicide for vegetation control in noncrop land. -
Definition
ChEBI: A diamino-1,3,5-triazine that is 1,3,5-triazine-2,4-diamine substituted by a chloro group at position 6 while one of hydrogens of each amino group is replaced respectively by an ethyl and a propan-2-yl group. -
Vorbereitung Methode
Atrazine is prepared by reacting cyanuric chloride with one equivalent of ethylamine, followed by one equivalent of isopropylamine in the presence of an acid-binding agent. -
Allgemeine Beschreibung
White crystalline solid. Melting point 173-175°C. Sinks in water. A selective herbicide used for season-long weed control in a variety of crops. -
Air & Water Reaktionen
Insoluble in water. -
Reaktivität anzeigen
Atrazine undergoes slow hydrolysis at 158° F under neutral conditions. Hydrolysis is more rapid in acidic or alkaline conditions. Forms salts with acids . -
Hazard
Hematologic, preproductive and develop- mental effects. Questionable carcinogen. -
Health Hazard
Acute oral toxicity of atrazine in experimen-tal animals was found to be moderate. Inhumans, the acute and chronic toxicity islow. There is no reported case of poisoning. The toxic symptoms in animals includeataxia, dyspnea, and convulsion. Other symptoms are abdominal pain, diarrhea, vomitingand irritation of mucous membranes. Theoral LD50 values in rats and rabbits are 672and 750 mg/kg, respectively (NIOSH 1986).
The toxicity signs reported in rabbitsincluded conjuctivitis, excessive salivation,sneezing, and muscle weakness (Salem et al1985a,b). There was a gradual reductionin the hemoglobin content and erythrocyteand leukocyte count; an increase in glucose,cholesterol, total proteins, and the enzymeserum transminases. The oral LD50 valuereported is 3320 mg/kg. Rabbits fed atrazine-treated maize for 6 months developed loss ofappetite, debility, progressive anemia, enteritis, and muscle weakness. Most organs wereaffected.
Rats treated orally with atrazine 12 mg/100 g for 7 days were found to contain theunchanged atrazine, as well as its metabolites in the liver, kidney, and brain. Thehighest concentration of unchanged atrazinewas detected in the kidney, while its majormetabolite, diethylatrazine, was found in thebrain (Gojmerac and Kniewald 1989). Whena single dose of atrazine (0.53 mg) was administered to rats by gavage, 15% of itwas retained in the body tissues, mostly inthe liver, kidneys, and lungs, and the restexcreted out in 72 hours.
Donna and coworkers (1986) conducteda 130-month study to test the carcinogenic-ity of atrazine in male Swiss albino mice.Intraperitoneal administration of a total doseof 0.26 mg/kg showed a statistically sig-nificant increase of plasma cell type andhistiocytic type of lymphomas in the ani-mals. IARC has listed atrazine as possiblycarcinogenic to human (Group 2B Carcinogen) (IARC 1991). Lifetime administrationof atrazine in rats caused mammary tumors.EPA has classified atrazine as a possiblehuman carcinogen. It produced severe eyeirritation in rabbit. Irritant action on skinis mild.
Atrazine has been detected in surface- andgroundwaters in many parts of the UnitedStates. The U.S. EPA has set the MCL(maximum contaminant level) for atrazinein the drinking waters as 3μg/L. In otherwords, the maximum permissible level inwater delivered to any user of a public watersystem must not be at this level or exceedthis level. -
Brandgefahr
Special Hazards of Combustion Products: Irritating hydrogen chloride and toxic oxides of nitrogen may be formed. -
Landwirtschaftliche Anwendung
Atrazine is the generic name for 2-chloro-4-ethylamino- 6-isopropylamino-s-triazine.A trazine is an example of photosynthesis inhibitors and herbicides.
Atrazine was the first s-triazine used in maize. The use of this herbicide and others in the same group has expanded to selective application in perennial crops and orchids as well as for non-crop and industrial sites. -
Landwirtschaftliche Anwendung
Herbicide, Plant growth regulator: Not approved for use in EU countries. A U.S. EPA restricted Use Pesticide (RUP). In 2009 a report from the Natural Resources Defense Council (NRDC) reported that atrazine is the most commonly detected pesticide in U.S. waters. Atrazine is a selective pre-and post-emergence herbicide used for the control of broadleaf and grassy weeds in crops, such as corn (field and sweet), guava, hay, macadamia nuts, range grasses for the establishment of permanent grass cover on range lands and pastures in Oklahoma, Nebraska, Texas and Oregon, wheat, residential and recreational turf and sod farms, sorghum, sugarcane, pineapples, and Christmas trees and ornamentals. It is also used in forestry and, at higher application rates, for non-selective weed control in non-crop areas. It is the most widely used pesticide in the United States. Use data from 1900 to 1997 indicate that approximately 76.5 million pounds of atrazine active ingredient is used domestically each year. Certified herbicide workers may spread atrazine on crops or crop lands as a powder, liquid, or in a granular form. Atrazine is usually used in the spring and summer months. For it to be active, atrazine needs to dissolve in water and enter the plants through their roots. It then acts in the shoots and leaves of the weed to stop photosynthesis. Atrazine is taken up by all plants, but in plants not affected by atrazine it is broken down before it can have an effect on photosynthesis. Atrazine degrades into hydroxy compounds and chlorotriazine degradates. The application of atrazine to crops as a herbicide accounts for almost all of the atrazine that enters the environment, but some may be released from manufacture, formulation, transport, and disposal. Atrazine does not tend to accumulate in living organisms such as algae, bacteria, clams, or fish, and, therefore, does not tend to build up in the food chain. Atrazine can be applied by ground boom sprayer, aircraft, tractor-drawn spreader, rights-of-way sprayer, hand-held sprayer, backpack sprayer, lawn handgun, pushtype spreader, and bellygrinder. -
Handelsname
AI3-28244®; AATRAM®; AATREX®; ACTINITE PK®; ACTINIT A®; AGIMIX® Atrazine; AKTIKON®; AKTIKON PK®; AKTINIT A®; ALAZINE®; ARGEZIN®; ATAZINAX®; ATERBUTEX®; ATERBUTOX®; ATLAS ATRAZINE®; ATLAZIN D-WEED®; ATRANEX®; ATRASINE®; ATRATAF®; ATRATOL®; ATRAZINEK®; ATRAZINE 90DF®; ATREX®; AXIOM® Atrazine; AZINOTOX®; BICEP®; BLADEX/ATRAZINE (2:1) 80 W®[C]; BUCTRIL + ATRAZINE GEL®[C]; CANDEX®; CEKUZINA-T®; CHROMOZIN®; CO-OP ATRAZINE®[C]; CRISATRINA®; CRISAZINE®; CYAZIN®; DOW ATRAZINE 80 W HERBICIDE®[C]; ERUNIT 500 FW®; FARMCO® ATRAZINE; FENAMIN®; FENATROL®[C]; FIELD MASTER®; FLOWABLE ATRAZINE®; G 30027®; GEIGY 30,027®; GESAPRIM®; GESOPRIM®; GRIFFEX®; GRIFFIN ATRAZINE 90 DRY FLOWABLE HERBICIDE®[C]; HAVILAND ATRAZINE LINURON WEED KILLER®[C]; HELENA ATRAZINE TECHNICAL®[C]; GUARDSMAN® herbicide (mixture of atrazine and di- methenamid); HELENA BRAND ATRAZINE®[C]; HERBATOXOL®; HERBIMIX SC®; HERBITRIN 500 BR®; HUNGAZIN®; INAKOR®; LADDOK®; LANCO ATRAZINE®[C]; LARIAT®; LEADOFF®; MAGIC CARPET FERTILIZER WITH ATRAZINE®[C]; MALLET PM BROMOXYNIL, ATRAZINE BROADLEAF HERBICIDE®[C]; MARKSMAN®; MARZONE ATRAZINE®[C]; MITAC®; NEW CHLOREA®; NU-TRAZINE 900 DF®; NU-ZINOLE AA®; OLEOGESAPRIM®; PATRIOT®; PITEZIN®; POSMIL®; PRIMATOP®; PRIMOLE®; PROKIL ATRAZINE 80 W®[C]; RADAZIN®; RADIZINE®; READY MASTER®; RESIDOX®; SHELL® ATRAZINE 80 W HERBICIDE[C]; SIMAZAT®; STRAZINE® TRIAZINE A 1294; TRIPART® ATRAZINE 50 SC; VECTAL®; WEEDEX®; WONUK®; ZEAZIN®; ZEAZINE® -
Pharmakologie
In plants, themajor pathways of atrazine transformation include hydroxylation, N-dealkylation, and glutathione conjugation. Hydroxylation of atrazine and other s-triazines occurs in a wide range of plants and is considered a detoxification mechanism because hydroxyatrazine is not phytotoxic. Hydroxylation is catalyzed via reaction with the naturally occurring compound, benzoxazinone (127). A hypothetical ether-linked intermediate has been proposed to undergoes hydrolysis to produce hydroxylated triazine and regenerated benzoxazinone. This reaction occurs in many susceptible and resistant plant species, and the rate of this reaction is governed by the amount of benzoxazinone present in the tissue. The hydroxylation reaction predominates in root tissue, whereas GSH conjugation is more prominent in leaf tissue of plants containing a GST that has substrate specificity for atrazine. Because atrazine is a photosystem II (PS II) inhibitor, possession of a rapid detoxification system, such as a specific GST, is paramount to provide tolerance to this compound. -
Sicherheitsprofil
Poison by intraperitoneal route. Moderately toxic by ingestion. Mildly toxic by inhalation and skin contact. An experimental teratogen. Other experimental reproductive effects. Human mutation data reported. A skin and severe eye irritant. Questionable carcinogen with experimental tumorigenic data. When heated to decomposition it emits toxic fumes of ClandNOx. -
mögliche Exposition
Atrazine is an herbicide and plant growth regulator used for season-long weed control in corn, sorghum, and certain other crops. Banned for use as a pesticide in the EU. US annual use . 75 millon pounds. -
Erste Hilfe
If this chemical gets into the eyes, remove anycontact lenses at once and irrigate immediately for at least15 min, occasionally lifting upper and lower lids. Seekmedical attention immediately. If this chemical contactsthe skin, remove contaminated clothing and wash immediately with soap and water. Seek medical attention immediately. If this chemical has been inhaled, remove from exposure, begin rescue breathing (using universal precautions, including resuscitation mask) if breathing hasstopped and CPR if heart action has stopped. Transferpromptly to a medical facility. When this chemical hasbeen swallowed, get medical attention. Give large quantities of water or milk and induce vomiting. Do not make anunconscious person vomit. -
Carcinogenicity
An increase in mammary adenomas and fibroadenomas was observed in female rats fed 1000 ppm, but not 500 ppm atrazine or less for their lifetime. An increase in the incidence of mammary carcinomas was seen at 70, 500, and 1000 ppm, but not at 10 ppm. The biological significance of these findings is not known but may be related to a hormonally mediated mechanism. Rats fed 375 or 750 ppmatrazine for 2 years showed an increase in mammary tumors in males at 750 ppm. Uterine carcinomas increased in both groups and the incidence of malignant tumors also increased in both sexes. -
Environmental Fate
Atrazine is highly persistent in soil. In soil and water, atrazine degrades by hydrolysis, followed by biodegradation by soil microorganisms. Hydrolysis is rapid in acidic or basic environments, but is slower around neutral pH. Sunlight and evaporation do not affect its removal rate. Atrazine can persist for longer than 1 year under dry or cold conditions. It is moderately to highly mobile in soils with low clay or low organic matter content. Because it does not adsorb strongly to soil particles and has a lengthy half-life (60 to >100 days), it has a high potential for groundwater contamination despite being only moderately soluble in water. It is frequently detected in drinking water wells. -
Lager
Color Code—Blue: Health Hazard/Poison: Store ina secure poison location. Prior to working with this chemicalyou should be trained on its proper handling and storage.Should be stored in tightly closed containers away fromstrong acids. -
Versand/Shipping
UN2763 Triazine pesticides, solid, toxic, Hazard Class: 6.1; Labels: 6.1-Poisonous materials. -
Toxicity evaluation
According to US EPA, the underlying mechanism for tumor induction in female SD rats involves a reduction in the release of gonadotropin releasing hormone (GnRH) from the hypothalamic– pituitary–gonadal axis in the rats, followed by attenuation of the afternoon pituitary LH surge, leading to a lengthening of the estrus cycle that increases estrogen levels that in turn is associated with an increased incidence of mammary tumors in SD rats. The decrease GnRH release follows these postulated events: hypothalamic changes results in an increase in the release of corticotropin releasing hormone (CRH), elevated CRH stimulates release of adrenocorticotropic hormone (ACTH) from the pituitary, elevated ACTH stimulates production of corticosterone and progesterone by the adrenal, and some or all of these events decrease GnRH release. Atrazine and some of its metabolites act to attenuate the spontaneous preovulatory LH surge, block the gonadal steroid inducted LH surge and attenuate concomitant GnRH neuronal activation, inhibit LH secretion, and increase the concentration of GnRH in the median eminence (a measure of reduced release). This mode of action (premature reproductive aging or senescence) hastens the onset of mammary gland tumors.
The central nervous system (CNS) mode of action that results in altered pituitary hormone function, especially LH and prolactin (PRL) secretions, occurs in both adults and the young. The triazine-mediated changes in the hypothalamus– pituitary–gonadal axis relating to neuroendocrine and neuroendocrine-related developmental and reproductive toxicity are considered relevant to humans. On the other hand, the exact mechanism by which the mode of action changes neurotransmitters and neuropeptides within the CNS is not understood. It was noted that although atrazine alters hypothalamic norepinephrine and dopamine, these effects do not necessarily represent its primary site of action, but that these CNS alterations may be a signal of potential upstream effects on other neurotransmitters. It is unclear if atrazine directly affects the hypothalamus, setting off the cascade of events, or affect indirectly through the hypothalamus–pituitary–adrenal axis or both -
Inkompatibilitäten
Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides. -
Waste disposal
Atrazine is hydrolyzed by either acid or base. The hydroxy compounds are generally herbicidally inactive, but their complete environmental effects are uncertain. However, the method appears suitable for limited use and quantities of triazine. Atrazine underwent .99% decomposition when burned in a polyethylene bag, and combustion with a hydrocarbon fuel would appear to be a generally suitable method for small quantities. Combustion of larger quantities would probably require the use of a caustic wet scrubber to remove nitrogen oxides and HCl from the product gases.
Atrazin Anbieter Lieferant Produzent Hersteller Vertrieb Händler.
Global(420)Suppliers
Region
-
Qingdao Trust Agri Chemical Co.,Ltd
Telefon +8613573296305
E-Mail aroma@qdtrustagri.com
-
Hebei Mojin Biotechnology Co.,Ltd
Telefon +8613288715578
E-Mail angelia@hbmojin.com
-
HEBEI SHENGSUAN CHEMICAL INDUSTRY CO.,LTD
Telefon +8615350851019
E-Mail admin@86-ss.com
-
Henan Tianfu Chemical Co.,Ltd.
Telefon +86-0371-55170693<br/>+86-19937530512
E-Mail info@tianfuchem.com
-
Telefon +8615858145714
E-Mail FandaChem@Gmail.com
-
Telefon +undefined-21-51877795
E-Mail ivan@atkchemical.com
-
Shanghai Zheyan Biotech Co., Ltd.
Telefon 18017610038
E-Mail zheyansh@163.com
-
Telefon +86-0371-86658258<br/>+8613203830695
E-Mail sales@coreychem.com
-
Hubei Jusheng Technology Co.,Ltd.
Telefon 18871490254
E-Mail linda@hubeijusheng.com
-
Hubei xin bonus chemical co. LTD
Telefon 86-13657291602
E-Mail linda@hubeijusheng.com
1of2
1912-24-9, Atrazine Verwandte Suche:
- Hydroxyethyl Cellulose
- 2-Piperazin-1-ylethylamin
- 6-Propyl-2-thiouracil
- 1,3,5-Triazin
- Ethyldiisopropylamin
- Ethyl cellulose
- Hydroxyethyl starch
- 3-Ethoxy-4-hydroxybenzaldehyd
- 2-Ethyl-3-hydroxy-4H-pyran-4-on
- 4,6-Diamino-1,3,5-triazin-2(1H)-on
1of4
