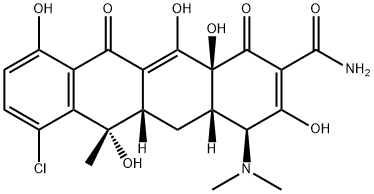
Chlortetracyclin
Bezeichnung:Chlortetracyclin
CAS-Nr57-62-5
Englisch Name:Chlortetracycline
CBNumberCB5408663
SummenformelC22H23ClN2O8
Molgewicht478.88
MOL-Datei57-62-5.mol
Synonyma
Chlortetracyclin
Chlortetracyclin physikalisch-chemischer Eigenschaften
| Schmelzpunkt | 168-169° |
| alpha | D23 -275.0° (methanol) |
| Siedepunkt | 821.1±65.0 °C(Predicted) |
| Dichte | 1.2833 (rough estimate) |
| Brechungsindex | 1.6000 (estimate) |
| storage temp. | 2-8°C |
| Löslichkeit | DMF: Soluble; DMSO: Soluble; Ethanol: Soluble; Methanol: Soluble |
| Aggregatzustand | A solid |
| pka | pKa 3.3 (Uncertain) |
| Wasserlöslichkeit | 0.63g/L(25 ºC) |
| LogP | -0.620 |
| CAS Datenbank | 57-62-5(CAS DataBase Reference) |
| EPA chemische Informationen | Chlortetracycline (57-62-5) |

