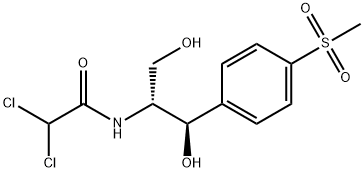
Thiamphenicol
Bezeichnung:Thiamphenicol
CAS-Nr15318-45-3
Englisch Name:Thiamphenicol
CBNumberCB0102588
SummenformelC12H15Cl2NO5S
Molgewicht356.22
MOL-Datei15318-45-3.mol
Synonyma
Thiamphenicol
Thiamphenicol physikalisch-chemischer Eigenschaften
| Schmelzpunkt | 163-166 °C |
| alpha | D25 +12.9° (ethanol) |
| Dichte | 1.3281 (rough estimate) |
| Brechungsindex | 1.6000 (estimate) |
| storage temp. | Sealed in dry,Room Temperature |
| Löslichkeit | ethanol: 50 mg/mL, clear, colorless |
| Siedepunkt | 695.9±55.0 °C(Predicted) |
| pka | 11.05±0.46(Predicted) |
| Aggregatzustand | powder |
| Farbe | white to off-white |
| Wasserlöslichkeit | Soluble in acetonitrile or DMF. Slightly soluble in water |
| Merck | 14,9301 |
| BRN | 2819542 |
| InChIKey | OTVAEFIXJLOWRX-NXEZZACHSA-N |
| CAS Datenbank | 15318-45-3 |
| EPA chemische Informationen | Thiamphenicol (15318-45-3) |
| S-Sätze: | 22-24/25 |
| WGK Germany | 2 |
| RTECS-Nr. | AB6680000 |
| HS Code | 29414000 |
| Toxizität | human,TDLo,unreported,214mg/kg/10D (214mg/kg),BEHAVIORAL: SLEEPGASTROINTESTINAL: NAUSEA OR VOMITINGSKIN AND APPENDAGES (SKIN): "DERMATITIS, OTHER: AFTER SYSTEMIC EXPOSURE",Arzneimittel-Forschung. Drug Research. Vol. 24, Pg. 944, 1974. |

