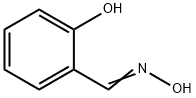
What is Salicylaldoxime?
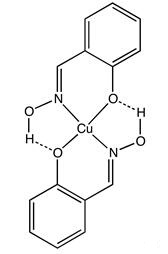
Scheme 1 Structure of the copper (II) complex of the conjugate base of salicylaldoxime
Salicylaldoxime is an organic compound described by the formula C6H4CH=NOH-2-OH and it is the oxime of salicylaldehyde. This crystalline, colorless solid is a chelator and sometimes used in the analysis of samples containing transition metal ions, with which it often forms brightly colored coordination complexes [1]. In the era when metals were analyzed by spectrophotometry, many chelating ligands were developed that selectively formed brightly colored complexes with particular metal ions. This methodology has been eclipsed by the introduction of the inductively coupled plasma methodology. Salicylaldoxime can be used to selectively precipitate metal ions for gravimetric determination.
L. P. Biefeld and W. B. Ligett studied the separation of copper from other metals which form complexes with salicylaldoxime because copper salicylaldoximate may be precipitated in weakly acidic solutions, whereas the salícylaldoximates of most other metals are precipitated only from neutral or slightly basic solutions. Lead has been separated from silver, zinc, and cadmium by precipitation in strongly ammoniacal solution. It studied the effect of pH on the precipitation of zinc salicylaldoximate, and to determine the best conditions for a separation of copper, lead, and zinc based upon pH control and ammonia-complex formation [2].
Salicylaldoxime is recognized as a selective precipitant for copper (scheme 1), although it forms water-insoluble chelates with a number of elements [3]. Ingvar Dahl invested the extraction of divalent magnesium, manganese, cobalt, nickel, copper, zinc, cadmium, mercury, and lead with solutions of salicylaldoxime in benzene and the effect of the reagent concentration and particularly the pH of the aqueous phase on the extractability was also studied. The solubility of salicylaldoxime in organic solvents has been utilized in the solvent extraction of carious elements. Subsequent determinations of the extracted metals have been accomplished either by optical methods, such as emission spectrography, colorimetry, absorption, and emission spectrophotometry and atomic absorption spectroscopy, or by radiometric measurements.
References
[1]https://en.wikipedia.org/wiki/Salicylaldoxime
[2]L. P. Biefeld and W. B. Ligett, Separation of Copper, Lead, and Zinc with Salicylaldoxime, Anal. Ed., 1942, 14, 4, 359-361
[3]Ingvar Dahl, The application of salicylaldoxime in solvent extraction, Analytica Chimica Acta
Volume 41, 1968, 9-14
You may like
Related articles And Qustion
See also
Lastest Price from Salicylaldoxime manufacturers

US $0.00/KG2023-09-05
- CAS:
- 94-67-7
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 50000KG/month

US $8.90/KG2023-01-10
- CAS:
- 94-67-7
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10 mt