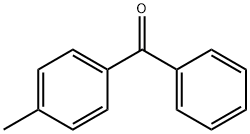
The toxicity of 4-Methylbenzophenone (HRcure-MBZ)
Introduction
4-Methylbenzophenone (HRcure-MBZ) and benzophenone are aromatic ketones with a molecular weight of 196 g/mol and 182 g/mol, respectively. 4-Methylbenzophenone differs from benzophenone only by a methyl group. They are strong UV light absorbers due to their conjugated structure, with a carbonyl group bridging two phenyl rings. Consequently, they are found to be used in sunscreen creams and as photoinitiators for polymerization reactions.

At the beginning of 2009, the German and Belgian authorities reported through RASFF (Rapid et al. for Food and Feed) that the photoinitiator 4-methylbenzophenone had been detected in cereal products. The estimated concentrations were as much as 4000 micrograms/kg. It was concluded that the contamination had derived from the transfer of 4-methylbenzophenone from the printed surface of the cardboard box, where it is used as a photoinitiator in the UV-hardened lacquer[1].
Uses
In the area of printing inks, HRcure-MBZ and benzophenone are used as photoinitiators for inks and lacquers that are cured with ultraviolet light. Benzophenone is widely used because it is relatively cheap and effective. UV-cured inks and lacquers are used without solvent; they typically contain 5–10% photoinitiator. 4-Methylbenzophenone may be used instead of benzophenone and, less frequently, in combination with benzophenone, as provided by the European Printing Ink Association. Photoinitiators are not completely used up or removed during or after printing, nor are they bound irreversibly into the print film layer. Due to their volatility, 4-methyl benzophenone and benzophenone may migrate through packaging to the food if no functional barrier, such as aluminum foil, is present. Internal plastic bags, used as a barrier to moisture, do not always act as a functional barrier. Specific migration studies have shown that benzophenone can migrate from paper and board into dry foods or powders, simulating dry foods. 4-Methylbenzophenone is also used as a constituent of synthetic perfumes and sunscreen agents to reduce skin damage to UV solar radiation[2].
Toxicity
The oral toxicity of both compounds is low as the rat LD50 is >10 g/kg b.w. and the mouse LD50 is 2.9 g/kg b.w. for benzophenone (EC, 2000) and >2 g/kg for 4-methylbenzophenone.
The in silico structure-activity evaluation of 4-methylbenzophenone (HRcure-MBZ) by the Toxtree v1.51 software developed by the European Commission Joint Research Centre does not highlight any structural alert for genotoxicity. Supportive evidence on the lack of genotoxicity of 4-methylbenzophenone can also be obtained from reading across from the structurally related benzophenone, for which adequate evidence of absence of genotoxicity is available. There is insufficient direct evidence available on the genotoxicity of 4-methylbenzophenone. However, based on structural considerations and experimental results on the structurally related benzophenone, it can be concluded that 4-methylbenzophenone does not raise concerns for genotoxicity.
References
[1] Lilianne Abramsson-Zetterberg, Kettil Svensson. “4-Methylbenzophenone and benzophenone are inactive in the micronucleus assay.” Toxicology letters 201 3 (2011): Pages 235-239.
[2] (EFSA), European Food Safety Authority. “EFSA statement on the presence of 4-methylbenzophenone found in breakfast cereals.” EFSA Journal 7 3 (2009).
You may like
See also
Lastest Price from 4-Methylbenzophenone manufacturers

US $0.00/Kg/Drum2025-04-21
- CAS:
- 134-84-9
- Min. Order:
- 1KG
- Purity:
- 99%min
- Supply Ability:
- 500kg

US $10.00/KG2025-04-21
- CAS:
- 134-84-9
- Min. Order:
- 100KG
- Purity:
- 99%
- Supply Ability:
- 100 mt