The wide range of applications: 1,1,1,3,3,3-Hexafluoro-2-propanol
Introduction
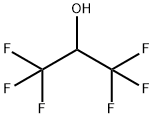
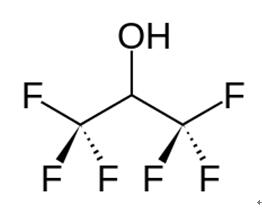
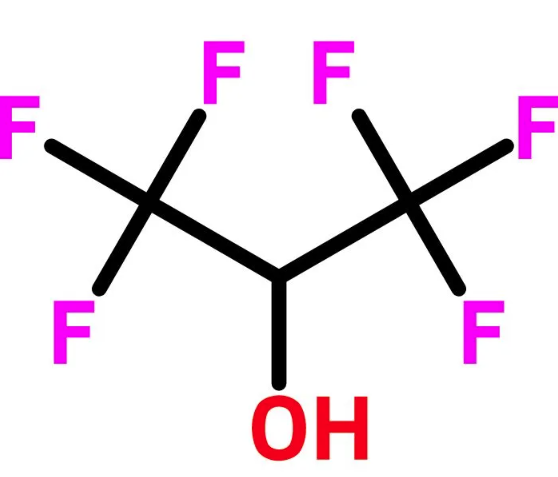
Hexafluoro-2-propanol (HFIP; 1,1,1,3,3,3-Hexafluoro-2-propanol) is a low-boiling solvent used in a variety of chemical applications. It first appeared in the chemical literature in a 1960 Swiss patent to Soviet Union chemists Ivan L. Knunyants and M. P. Krasuskaya, who synthesized it by reducing hexafluoroacetone with sodium borohydride.
In organic chemistry
Currently, the use of 1,1,1,3,3,3–hexafluorisopropan–2–ol (HFIP) as solvent in different areas of chemistry has had an increase, since it has both physical and chemical properties that allow obtaining unique modes of reactivity of molecules and reactive intermediaries. This reactivity is caused by the acid-base or electronic properties of the solvent. The use of HFIP has been reported in various reactions in organic chemistry, such as: in the activation of hydrogen peroxide to carry out epoxidation reactions of alkenes, Baeyer-Villiger oxidation of ketones, intramolecular Schmidt reaction, Friedel-Crafts reaction of acyl chlorides, Hosomi-Sakurai allylation of dimethyl acetals and allylic substitution of alcohols. In all of them, the hydrogen bond donating ability of HFIP allows the activation of the functional groups without the need to use catalysts or any additive. The addition of an acid promoter (organic acid) increases the Brønsted acid reactivity favoring these chemical processes[1].
Electro- and photoredox catalysis
The remarkable solvent effect of HFIP is not constrained only to the conventional synthetic platform, rather it is quite impressing in electrocatalysis as well as photocatalysis. Excellent redox stability in combination with a high dielectric constant (ε, 15.7) makes HFIP perfect for photoredox chemistry. The superpolar nature of the solvent [102 factor higher than trifluoroacetic acid (TFA) and by a factor of ∼108 compared to acetonitrile (MeCN)] assists in stabilizing radical cations, which are a frequent intermediate in electro- and photoredox catalysis. Probably, due to the same reason, the solvent is found to be way more efficient than any other polar solvents in a recent δ-C–H heteroarylation of protected aliphatic amines via [1,5]HAT[2]. Because of the high reactivity of the radical cations, the whole catalysis predominantly depends on the reaction media. It has been observed that non-nucleophilic HFIP can easily solvate the counter anions by H-bonding interactions, thus leaving the radical cations free.
Biological experiments
Apart from typical synthetic chemical transformations, HFIP is very common in Biological experiments. The effects of alcoholic solvents on proteins and peptides, to find out the cause of their stability inside the biological system, are a topic of great importance. Seminal studies indicate two major causes of alcohol-mediated protein denaturation (a) disruption of the natural state and (b) introduction of an α-helical motif. Polar and protic alcoholic solvents disrupt the distal hydrophobic interactions and elevate the local polar or hydrophilic interactions via weak interactions such as H-bonding. It is worth mentioning that HFIP with six fluorine atoms significantly induces α-helical conformation and imposes local polar interactions to a higher extent, which leads to protein denaturation. Additionally, hexafluoroisopropanol indirectly influences the structural modification of protein and lipid layers of different biological membranes. Apart from peptide chemistry, HFIP is potential enough to produce lipid bilayer leakage and alter the biochemical properties of the lipid phase.
References
[1] José Manuel Ramos-Villaseñor. “Review—Use of 1,1,1,3,3,3–hexafluoro–2–propanol (HFIP) Co-Solvent Mixtures in Organic Electrosynthesis.” Journal of The Electrochemical Society (2020).
[1] Bhattacharya, Trisha Animesh Ghosh and Debabrata Maiti. “Hexafluoroisopropanol: the magical solvent for Pd-catalyzed C–H activation.” Chemical Science 11 (2021): 3857–3870.
You may like
Related articles And Qustion
Lastest Price from 1,1,1,3,3,3-Hexafluoro-2-propanol manufacturers
US $0.00/KG2025-11-29
- CAS:
- 920-66-1
- Min. Order:
- 2000KG
- Purity:
- 99.9%
- Supply Ability:
- 20tons
US $1.00/KG2025-04-21
- CAS:
- 920-66-1
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10 mt