N-Hydroxysuccinimide and N-hydroxysuccinimide ester
Introduction
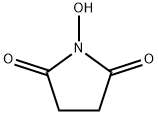
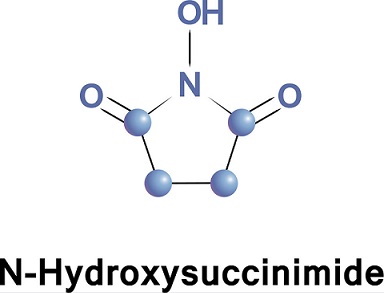
N-Hydroxysuccinimide, commonly known as NHS, is an intermediate used in peptide chemistry to prepare various activated esters of acylamino acids. It is a hydroxylated succinimide widely used in activating carboxylic acids to NHS-esters to promote coupling with nucleophiles. This approach is most commonly employed in activating the free acid forms of fluorophores to promote the conjugation of these fluorophores to amine residues of proteins. NHS ester activation has also been described in synthesizing N-acyl amino acids.
More than 50 years ago, N-hydroxysuccinimide (NHS) was proposed by the Anderson group for the preparation of active esters. These compounds are extremely useful for peptide synthesis and preparing all kinds of bioconjugates. The favorable reactivity of NHS esters, their relative stability towards hydrolysis, good crystallizability, and low toxicity have made NHS esters some of the most widely used chemical reagents.
Preparation of N-hydroxysuccinimide ester
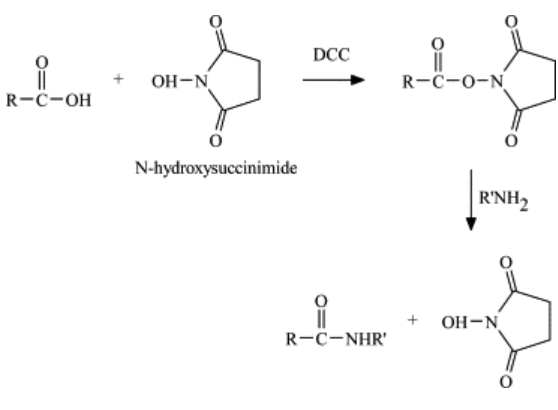
The formation of an N-hydroxysuccinimide ester by reaction of the carboxylic acid group with N-hydroxysuccinimide (NHS), frequently in the presence of dicyclohexylcarbodiimide, is another carboxylic acid mediated reaction, and one that is frequently used in preparing conjugates for contaminant immunoassays. The advantage of this reaction is that the active ester is reasonably stable under acidic conditions, so purification and storage of the active ester is possible. The active ester of NHS reacts quickly with amino groups of protein, resulting in good yields (i.e., higher epitope density). Although some loss of enzymatic activity during coupling has been reported, this reaction is less severe on the structure of the protein than the carbodiimide reaction. In a comparison of the carbodiimide reaction and NHS reaction for coupling of atrazine derivative to the enzymes, alkaline phosphatase, and horseradish peroxidase, it was evident that the NHS reaction resulted in an enzyme-conjugate with a higher epitope density and immunoreactivity than the enzyme-conjugate prepared by the carbodiimide reaction.
The uses of N-Hydroxysuccinimide esters
N-Hydroxysuccinimide (NHS) esters are widely used to conjugate proteins to peptides or to label (e.g., fluorescent tag) a protein or peptide. NHS esters are capable of reacting with amines under mild alkaline pH conditions without carbodiimide activation. Furthermore, free amino groups (e.g., serine, tyrosine, threonine hydroxyl) in proteins or peptides can also react with NHS esters. The conjugation conditions of an NHS ester involve organic solvents, such as dimethyl formamide (DMF), dichloromethane (DCM), or dimethyl sulphoxide (DMSO), which can destroy the stability and functionality of proteins/peptides, and they lead to the poor control of stoichiometry of NHS ester. Although N-terminal protein/peptides can be conjugated with NHS esters for protein/peptide labeling for biochemical analysis, the NHS ester reaction is not a common method used in vaccine bioconjugation due to the high concentration of organic solvent required during the reaction, leading to an increased potential of toxicity in the final vaccine design.
References
[1] Kung-Tsung Wang, Boris Weinstein, Donald N. Brattesan. “Heterocyclic compounds. VI. An improved preparation of n-hydroxysuccinimide†.” Journal of Heterocyclic Chemistry 3 1 (1966): 98.
[2] Lee, N. and I. Kennedy. “Chapter 5 – Immunoassays.”Food Toxicants Analysis (2007): 91-145.
You may like
Related articles And Qustion
See also
Lastest Price from N-Hydroxysuccinimide manufacturers

US $10.00/KG2025-04-21
- CAS:
- 6066-82-6
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10 mt

US $0.00/Kg/Drum2025-04-21
- CAS:
- 6066-82-6
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 500kg