N-Hydroxysuccinimide: Synthesis, application and pharmacokinetics
General description
N-Hydroxysuccinimide (NHS) is an organic compound with the formula (CH2CO)2NOH. It is a white solid that is used as a reagent for preparing active esters in peptide synthesis. It can be synthesized by heating succinic anhydride with hydroxylamine or hydroxylamine hydrochloride [1]. More than 50 years ago, N-Hydroxysuccinimide was proposed by the group of Anderson for the preparation of active esters. Its appearance is as follows:

Figure 1 Appearance of N-Hydroxysuccinimide.
Chemical synthesis
General One Pot Procedure for the Synthesis of N-Hydroxyimides [2]. To a suspension of imide (10 mmol) in 5ml of acetonitrile at room temperature were added di-tert-butyldicarbonate (1.1-2.2 eq) followed by DMAP (1-10mole%). Evolution of CO2 was accompanied by the dissolution of the imide. In some cases, the N-Boc derivative crystallized from the reaction medium. Progress of the reaction was monitored by TLC. When no starting material remained, an aqueous solution of hydroxylamine (0.61 ml of a 50 wt % aqueous solution, 10 mmol) was added. After stirring the mixture overnight at room temperature, addition of 10 ml of ether led to the precipitation of most of the hydroxylammonium salt of the N-hydroxyimide. The solid was filtered, washed thoroughly with ether and dried. It was next dispersed in 15 ml of water and diluted HCl was added until pH 1.
N-Hydroxyimides of low solubility in aqueous media were filtered, washed with water and dried. In the case of more water-soluble N-hydroxyimides, the aqueous phase was saturated with NaCl and extracted several times with ethyl acetate. The combined organic extracts were dried over Na2SO4 and the solvent removed under reduced pressure. N-Hydroxyimides so obtained were generally of sufficient purity for most synthetic purposes. Analytically pure samples were obtained after recrystallisation from the suitable solvent or sublimation in vacuum. N-Hydroxysuccinimide Colourless crystals, Yield 0.95 g, 93%; mp 95-96°C, after recrystallisation from ethyl acetate.
Application
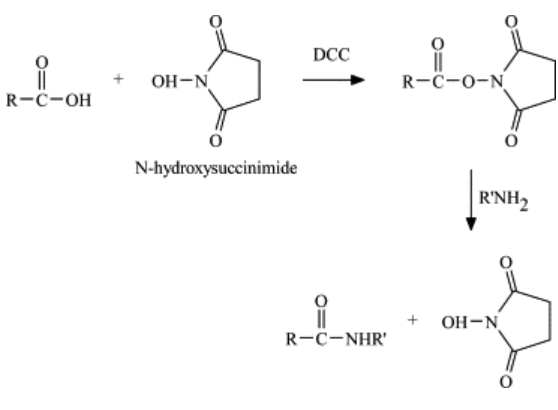
N-Hydroxysuccinimide is commonly found in organic chemistry or biochemistry where it is used as an activating reagent for carboxylic acids [3]. Activated acids (carboxylates) can react with amines to form amides for example, whereas a normal carboxylic acid would just form a salt with an amine. N-Hydroxysuccinimide esters are commonly used for protein modification (e.g. an N-Hydroxysuccinimide ester of fluorescein is commercially available, and can be added to a protein to obtain a fluorescently labeled protein in one simple reaction and purification step).
Determination method
The N-Hydroxysuccinimide can be determined by a HPLC method according to the previous work [4]. A zwitterionic silica-based HILIC column (Thermo Syncronis HILIC, 150 mm × 3 mm, 3 mm) was used for the isocratic separation of N-Hydroxysuccinimide samples in a 10 mM ammonium acetate buffer (pH 7.5). The mobile phase consisted of 90% acetonitrile and 10% of 10 mM aqueous ammonium acetate (pH 7.5 before mixing). The column temperature was set to 30 ℃. The system was equilibrated for 10 minutes before each run. The flow rate was 0.4 mL/min and the injection volume was 1 mL. Detection was performed by UV at 220 and 260 nm; and the total run time was 20 min. The detection limit was defined as a signal exceeding 3 times the noise of the baseline and the limit of quantitation as 6 times the noise (rounded values).
Pharmacokinetics
Three healthy volunteers were given 10 g of N-Hydroxysuccinimide in solution (100 ml). The subjects had fasted overnight. Blood samples of 3 ml were taken before the administration of N-Hydroxysuccinimide and at intervals after administration [5]. The blood concentration-time curve after oral administration of 3 g/75 kg of N-Hydroxysuccinimide every 8 h was therefore calculated. At steady state, the values for Cmax = 0.110 mg/ml and Cmin = 0.06 mg/rnl were reached only after the seventh dose. Theoretically, these limiting values could be obtained after one dose interval (8 h) by giving a loading dose of 5.76 g/75 kg, and could be sustained by giving a maintenance dose of 3.0 g every 8 h.
References
[1]Knight . N-Hydroxysuccinimide. Encyclopedia of Reagents for Organic Synthesis. 2001, 3(4): 55-61.
[2]Einhorn et al. Mild and convenient one pot synthesis of N-hydroxyimides from N-unsubstituted imides. Synthetic Communications, 2001, 31(5), 741-748.
[3]Anderson et al. N-Hydroxysuccinimide Esters in Peptide Synthesis. J. Am. Chem. Soc. 1963, 85 (19): 3039.
[4]Klykov et al. Quantification of N-hydroxysuccinimide and N- hydroxysulfosuccinimide by hydrophilic interaction chromatography (HILIC). Analytical Methods, 2015, 7, 6443–6448.
[5]Schulte et al. Pharmacokinetics of orally administered succinimide in man. European Journal of Drug Metabolism and Pharmacokinetics, 1978, 3: 161–163.
You may like
Related articles And Qustion
Lastest Price from N-Hydroxysuccinimide manufacturers

US $10.00/KG2025-04-21
- CAS:
- 6066-82-6
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10 mt

US $0.00/Kg/Drum2025-04-21
- CAS:
- 6066-82-6
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 500kg