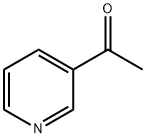
The Application and Synthesis of 3-Acetylpyridine
3-Acetylpyridine is a pyridine derivative and it is primarily used as a raw material or intermediate for synthesizing various therapeutic drugs In the pharmaceutical field.
3-Acetylpyridine serves as a key intermediate in the synthesis of risedronate sodium, an anti-osteoporosis drug. Osteoporosis is a systemic metabolic disorder characterized by reduced bone mass, deterioration of bone microarchitecture, increased bone fragility, and susceptibility to fractures. It has become a common and frequently occurring disease among the elderly, particularly postmenopausal women. Risedronate sodium, a third-generation bisphosphonate bone resorption inhibitor, is clinically used for the treatment and prevention of osteoporosis in postmenopausal women.
Furthermore, 3-acetylpyridine is an important raw material for the synthesis of imatinib mesylate. Imatinib mesylate was developed by Novartis AG, Switzerland and is used for the treatment of chronic myeloid leukemia and gastrointestinal stromal tumors.
The Synthesis of 3-acetylpyridine
1. Synthetic method 1
12.3 g of nicotinic acid was combined with 110 g of ethyl acetate. The mixture was stirred and cooled to 3°C, followed by the addition of 8.85 g of sodium ethoxide. After uniform stirring, 0.12 g of TiO₂ was added. The reaction mixture was heated to 53°C and maintained at this temperature for 3 hours.An intermediate control sample was analyzed by HPLC, confirming ethyl nicotinate content of 99.70%. The reaction was deemed complete. The mixture was cooled to 3°C, followed by the addition of 55 g of ethyl acetate and 11.56 g of sodium ethoxide. The reaction was heated to reflux at 78°C and maintained for 5 hours.The mixture was cooled to 5°C, and 50 mL of water was added. Subsequently, 85 g of hydrobromic acid was added dropwise. The reaction was heated to reflux and maintained for 5 hours. After cooling to room temperature, a sodium carbonate solution was added dropwise to adjust the pH to 9. The mixture was extracted with dichloromethane, and the dichloromethane layer was dried. Vacuum distillation was performed to collect the fraction boiling at 100-110°C under -0.095 MPa, and 11 g of 3-acetylpyridine was obtained with a purity of 98.7% and a molar yield of 90%.[1]
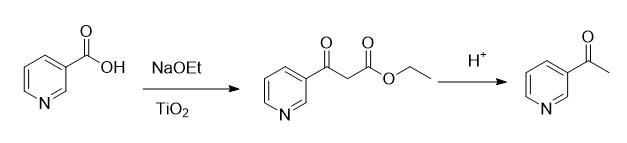
Fig.1. Synthesis of 3-acetylpyridine by nicotinic acid
2. Synthetic method 2
step 1: In a 1000 mL three-necked round-bottom flask, 3-bromopyridine (49.8 g, 0.31 mol), Pd(PPh₃)₂Cl₂ (5.4 g), and CuI (1.5 g) were added. The system was purged with nitrogen gas. Under controlled temperature (30°C) and stirring, diisopropylamine (510 mL) and trimethylsilylacetylene (34.2 g, 0.34 mol) were added sequentially. The reaction mixture was maintained at 30°C for 3 hours. Upon completion, the reaction was quenched with water, extracted with dichloromethane, and dried over MgSO₄. The solvent was removed under reduced pressure to obtain a concentrated intermediate.Subsequently, in a nitrogen-protected 1000 mL three-necked round-bottom flask, the concentrated intermediate was combined with KOH (19.2 g, 0.62 mol), methanol (570 mL), and dichloromethane (300 mL). The mixture was maintained at room temperature for 3 hours. After completion, the reaction was quenched with water, extracted with dichloromethane, and dried over MgSO₄. The solvent was removed under reduced pressure to yield 3-ethynylpyridine as a yellow solid (30.3 g, 93% yield), with a melting point of 38.5-39.5°C.
step 2: In a 250 mL round-bottom flask, 3-ethynylpyridine (28.8 g, 0.28 mol), water (10.2 g, 0.56 mol), trifluoromethanesulfonic acid (8.4 g, 0.56 mol), and trifluoroethanol (100 mL) were added sequentially. The flask was sealed and stirred at room temperature for 45 hours. Upon completion, the reaction mixture was transferred to a 500 mL separatory funnel, and ethyl acetate (250 mL) was added. The organic layer was washed sequentially with 100 mL of 1 M sodium bicarbonate solution and 100 mL of sodium chloride solution, dried over anhydrous magnesium sulfate, filtered, and concentrated under reduced pressure. Distillation yielded 3-acetylpyridine as a colorless oily liquid (32.19 g, 95% yield), with a boiling point of 218-219°C.[2]
3. Synthetic method 3
A mixture of 113.5 g (1 mol) of 3-chloropyridine and 45.1 g (1.1 mol) of acetonitrile (freshly distilled) is added dropwise to a suspension of 13.8 g (2.0 mol) of lithium granules in 350 ml of THF at -75°C over 2 hours. After reaching a conversion determined by GC of > 95% (overall 17.5 h), the reaction mixture is worked up repeatedly in the described manner. After filtration of the remaining toluenic solution over Primisil (high-density zeolite) and the condensing of the solvent. to give 110 g of 3-acetylpyridine (HPLC purity 93%).[3]
References
[1]Hu, Jiantao, Synthesis of 3-acetylpyridine via transesterification and condensation, [P] CN109053554.
[2]GUAN Yueqing, Synthesis of 3-acetylpyridine as a key intermediate for risedronate sodium, CHEMICAL RESEARCH, 2017,28(2), 191.
[3]Meudt, Andreas, Process for the preparation of substituted aromatics via lithiation and electrophilic alkylation of haloaromatics, [P] EP1270535.
You may like
See also
Lastest Price from 3-Acetylpyridine manufacturers

US $10.00/KG2025-04-21
- CAS:
- 350-03-8
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 100 mt
US $0.00-0.00/KG2025-04-04
- CAS:
- 350-03-8
- Min. Order:
- 1KG
- Purity:
- 98%
- Supply Ability:
- 1ton