Salicylaldoxime: Applications in Solvent Extraction and Synthesis Method
General Description
Salicylaldoxime, a compound derived from salicylaldehyde and hydroxylamine, holds significant importance in chemistry, medicine, and metallurgy. Its chelating properties enable stable complex formation with metal ions, crucial for industrial processes like metal extraction and purification. In medicine, it shows promise for treating neurodegenerative disorders due to its antioxidant and anti-inflammatory properties. Additionally, salicylaldoxime plays a vital role in solvent extraction, selectively isolating metals from complex mixtures with high efficiency. Its synthesis involves magnesium-mediated ortho-specific formylation of phenols, followed by conversion to salicylaldoxime through reaction with hydroxylamine sulfate. Overall, salicylaldoxime's versatility and effectiveness make it invaluable across various fields.

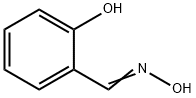
Figure 1. Salicylaldoxime
Overview
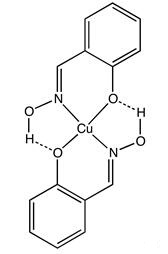
Salicylaldoxime, derived from salicylaldehyde and hydroxylamine, is a compound with wide-ranging significance in chemistry, medicine, and metallurgy. This organic compound is valued for its chelating properties, forming stable complexes with metal ions, particularly copper, nickel, and cobalt, which are essential in industrial processes like metal extraction and purification. In coordination chemistry, it serves as a key ligand, forming stable complexes with various metal ions. In medicine, studies suggest salicylaldoxime possesses antioxidant, anti-inflammatory, and neuroprotective properties, potentially useful in treating neurodegenerative disorders. These medicinal properties are currently being explored for their therapeutic applications. In metallurgy, salicylaldoxime plays a vital role in the extraction of metals from ores through solvent extraction. It forms complexes with metal ions in ore solutions, enabling the selective extraction and separation of specific metals, a crucial process in metallurgical industries. Salicylaldoxime's versatility and unique properties make it a valuable compound in diverse fields, including chemistry, medicine, and metallurgy. Ongoing research aims to further explore its potential applications and benefits in these areas. 1
Applications in solvent extraction
Salicylaldoxime, a versatile organic compound, plays a crucial role in solvent extraction processes due to its unique chemical properties. This compound is adept at binding with specific metal ions, making it invaluable for separating metals from complex mixtures. In solvent extraction, salicylaldoxime acts as a chelating agent, forming stable complexes with the target metals. This ability ensures the precise isolation of desired metals while minimizing contamination from other components. One of the key advantages of using salicylaldoxime in solvent extraction is its selectivity, which enables efficient metal separation. Additionally, its solubility characteristics in various solvents make it an ideal choice for different extraction techniques, further enhancing its versatility. By facilitating metal extraction and purification processes, salicylaldoxime not only improves efficiency but also contributes to environmental sustainability by reducing the reliance on harmful chemicals. The widespread application of salicylaldoxime in industrial processes underscores its reliability and effectiveness in metal extraction. Its role in solvent extraction showcases its significance in enhancing process efficiency, achieving precise metal separations, and promoting sustainable practices in the industry. 2
Synthesis
The synthesis of Salicylaldoxime involves a series of steps, primarily mediated by magnesium, yielding a high overall yield of 88%. Initially, phenols undergo deprotonation facilitated by magnesium methoxide. The free methanol generated during this process is subsequently removed through distillation. Paraformaldehyde is then added, leading to ortho-specific formylation under the influence of magnesium, resulting in the formation of salicylaldehyde magnesium salts. Acidic work-up allows for the isolation of salicylaldehydes from these salts. Alternatively, the synthesis route offers another pathway wherein aqueous hydroxylamine sulfate is added directly to the salicylaldehyde magnesium salts, bypassing the acidic work-up step. This modification leads to the formation of salicylaldoximes. Throughout the process, various solvents such as methanol, toluene, and water are utilized to facilitate different stages of the reaction. Methanol and toluene are used as solvents during the initial deprotonation and formylation steps, while water serves as the medium for the addition of hydroxylamine sulfate. In summary, the synthesis of Salicylaldoxime involves magnesium-mediated ortho-specific formylation of phenols followed by subsequent conversion to salicylaldehyde magnesium salts, which can be further transformed into salicylaldoximes through reaction with hydroxylamine sulfate. 3
Reference
1. Salicylaldoxime. National Center for Biotechnology Information. 2024; PubChem Compound Summary for CID 135408751.
2. Zhu K, He X, Chen P, Peng J, Dong X, Zhai S. Highly efficient Cu(II) capture by salicylaldoxime functionalized magnetic polydopamine core-shell hybrids: Behavior and mechanism. Int J Biol Macromol. 2024: 130549.
3. Aldred R. Magnesium-mediated ortho-specific formylation and formaldoximation of phenols. Journal of The Chemical Society-perkin Transactions 1. 1994; 24(1): 1823-1831.
Related articles And Qustion
Lastest Price from Salicylaldoxime manufacturers

US $7.00/kg2025-04-21
- CAS:
- 94-67-7
- Min. Order:
- 1kg
- Purity:
- 0.99
- Supply Ability:
- 10000

US $8.90/KG2025-04-21
- CAS:
- 94-67-7
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10 mt