What is 2-Hydroxyethyl Methacrylate?
2-hydroxyethyl methacrylate (HEMA) is a commercially important and widely used monomer. It is commonly prepared in a one-step reaction from methyl methacrylate or methacrylic acid. Specifically, HEMA can be synthesized by the following two methods: i. the first method involved the transesterification of ethylene glycol1; ii. the second is the reaction between ethylene oxide and methacrylic acid2. Several procedures were developed in order to remove the impurities in the production of HEMA, such as soaking, extraction and ion-exchange.3

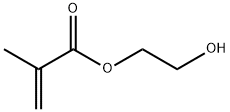
Fig 1. Chemical structure formula of 2-Hydroxyethyl Methacrylate

Fig 2. Synthesis methods of HEMA
As the major methacrylic derivative, HEMA can be polymerized by radical initiators or by various methods (γ-ray, UV, plasma, et. al). Its primary –OH group allows the substitution reactions with the monomer or the corresponding polymer4. By adopting various techniques, the grafting of p(HEMA) and copolymers prepared with HEMA as a comonomer has been performed with natural polymers such as cellulose, dextran, and starch. 5, 6 In addition, synthetic polymers, polyethylene, polyurethanes, poly vinylic alcohol, blends of acrylic networks and polyvinylic alcohol, and polyesters also give grafting reactions whose aim is to improve the mechanical and physical properties of the initial products.7-9
Poly(2-hydroxyethyl methacrylate) (p(HEMA)) is inert, water-stable, and nondegradable with high transparency. Because of its hydroxyethyl pendant groups, pHEMA is widely prepared in the form of hydrogel to manufacture soft contact lenses.10 Hydrogels generally absorb a large amount of water, and this swelling is responsible for the rubbery and soft properties of hydrogel. Hydrogels have found applications in environmental, biomedical, food, etc., fields. The physical properties of pHEMA (e.g., swelling, stiffness, and rheology) can be tuned by varying cross-linking density, incorporating different chemistries through copolymerization, and introducing mesoscopic pores. Specifically, a reduction in cross-linking density results in a softer, more malleable hydrogel that may be better suited for soft tissue regeneration. Moreover, copolymerization with acetic acid, methylmethacrylate, or dextran can adjust the permanence, hydrophilicity, and cellular adhesion in vivo. Finally, the introduction of mesoscopic porogens can facilitate vascular ingrowth, improve cellular attachment, and overcome limited permeability. Although pHEMA is considered nondegradable (which makes it ideally suited for long-term applications in vivo), degradable pHEMA copolymers have been fabricated by the integration of enzymatically susceptible monomers (e.g., dextran) or cross-linking agents. These degradable materials show promise for controlled release of pharmaceuticals and proteins.11
P(HEMA) is a well-known biocompatible product of high interest for medical applications in dentistry, bone cements, and biomaterials. Some of the main areas of interest for p(HEMA) have been listed below:
Optical lenses: the main application of p(HEMA) hydrogels is the preparation of contact and intraocular lenses used after cataract extraction. Also, the vision decrement associated with deposit accumulation on hydroxyethyl methacrylate (HEMA) contact lenses was assessed. 12, 13
Dentistry: p(HEMA) was found to be highly biocompatible and resorbable for primary teeth endodontic filling. However, due to its hydrophilicity, p(HEMA) appeared more useful in dentistry as a bonding reagent between dentine and other types of restorative resions.14, 15
Drug delivery: pilocarpine was trapped in a matrix diffusion-controlled drug delivery system using hydrophilic inserts of p(HEMA) to ensure an increased bioavailability of pilocarpine and prolong the length of time in which the medication remains in the eyes of the test subjects.16, 17
References
1. Kopeček, J.; Jokl, J.; Lím, D. In Mechanismus der dreidimensionalen Polymerisation von Glykolmethacrylaten, Journal of Polymer Science Part C: Polymer Symposia, Wiley Online Library: 1967; pp 3877-3889.
2. Montheard, J.-P.; Chatzopoulos, M.; Chappard, D., 2-hydroxyethyl methacrylate (HEMA): chemical properties and applications in biomedical fields. Journal of Macromolecular Science, Part C: Polymer Reviews 1992, 32 (1), 1-34.
3. Montheard, J.-P.; Chatzopoulos, M.; Chappard, D. J. J. o. M. S., Part C: Polymer Reviews, 2-hydroxyethyl methacrylate (HEMA): chemical properties and applications in biomedical fields. 1992, 32 (1), 1-34.
4. Nemours, E. d. P. d., Methacrylate resins. Industrial Engineering Chemistry 1936, 28 (10), 1160-1163.
5. Bamford, C. H.; Middleton, I. P.; Al-Lamee, K. G., Studies of the esterification of dextran: Routes to bioactive polymers and graft copolymers. Polymer 1986, 27 (12), 1981-1985.
6. Sandle, N.; Singh, O. P.; Varma, I. K., Graft copolymerization of starch with alkylene methacrylates. Angew. Makromol. Chem. 1987, 154 (1), 87-97.
7. Egboh, S. H., Free radical grafting of some hydrophilic monomers onto unsaturated segmented polyurethanes—a kinetic study. Angew. Makromol Chem., 1988, 156 (1), 151-162.
8. Moshonov, A.; Avny, Y., The initiation of in situ polymerization of vinyl monomers in polyester by glow discharge. J. Appl. Polym. Sci. 1980, 25 (1), 89-100.
9. https://pubchem.ncbi.nlm.nih.gov/compound/13360
10. https://pubs.acs.org/doi/10.1021/cm010939z
You may like
Related articles And Qustion
Lastest Price from 2-Hydroxyethyl methacrylate manufacturers

US $0.00/kg2025-06-11
- CAS:
- 868-77-9
- Min. Order:
- 190kg
- Purity:
- 90%
- Supply Ability:
- 20 MT

US $0.00/Kg/Drum2025-04-21
- CAS:
- 868-77-9
- Min. Order:
- 1KG
- Purity:
- 98.5%min
- Supply Ability:
- 20 tons