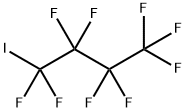
Perfluorobutyl Iodide: A Versatile, Environmentally Friendly Chemical with Low Toxicity
Perfluorobutyl iodide (PFBI) is a promising alternative to chlorofluorocarbon solvents used in aircraft ground maintenance operations and other military and commercial operations because it cleans well, has zero ozone depletion potential, and has extremely low global warming properties.

Activation of Perfluorobutyl iodide by anions
Developing new activation modes for carbon–halogen bonds of fluoroalkyl halides offers interesting possibilities for the synthesis of pharmaceuticals, agrochemicals, and new materials. Unlike typical alkyl halides, halogen atoms linked to strongly electron-withdrawing groups possess an electrophilic character, the so-called σ hole, which is opposite to that for R–X bonds. Recently, Chen, Melchiorre, Yu, and other groups showed that Perfluorobutyl iodide (Rf–I) represent valuable H–X donors, which can be activated by amines (HX acceptor) under light irradiation. Thereby perfluoroalkyl radicals are generated, which subsequently can be added to various electron-rich π bonds to give perfluoroalkylated products. Most recently, Niu and co-workers also discovered that allyl glycosyl sulfones can form halogen bond complexes with Perfluorobutyl iodide, which, by means of visible light irradiation, fragment via radical intermediates to give synthetically valuable glycosyl iodides. Despite these interesting results, activation of fluoroalkyl halides by different anions has been largely ignored in organic synthesis. Much to our surprise, the amination of THF with benzimidazole under standard reaction conditions not only gave the desired aminated product 17 in 59% isolated yield but also led to the iodinated product of compound 17.[1]
Apparently, in addition to the perfluoroalkyl radical, an iodine radical could be also be produced in our reaction system. This should allow selective iodination of heteroarenes with Perfluorobutyl iodide under similarly mild conditions to be achieved. In conclusion, we have developed versatile reaction systems for the amidation of alkyl ethers and benzylic hydrocarbons, for α-C(sp3)–H bond amidation, (hetero)arenes C–H iodination, and addition of Perfluorobutyl iodide to electron-rich π bonds. All these different synthetic protocols rely on activation of it by simple inorganic bases, such as sodium butoxide or potassium hydroxide. Mechanistic studies suggest that halogen bond interactions between it and the inorganic base promote the homolysis of Perfluorobutyl iodide. Notably, there is no requirement for expensive photoredox catalysts or transition metals, and the generated perfluoroalkyl radicals act as a hydrogen abstractor, iodine source, or perfluoroalkyl source for the synthesis of hemiaminal ethers, N-benzyl-arenesulfonamides, heteroaryl iodides, and perfluoroalkyl-substituted, and potentially bioactive, molecules. In addition, the practicality, easy scale up, and mild reaction conditions make these synthetic transformations attractive and valuable for organic synthesis.
Perfluorobutyl iodide: acute toxicity, subchronic toxicity and genotoxicity evaluations
Perfluorobutyl iodide is a promising alternative to chlorofluorocarbon solvents used in aircraft ground maintenance operations and other military and commercial operations, because it cleans well, has zero ozone depletion potential, and has extremely low global warming properties. Toxicity tests were performed with Perfluorobutyl iodide to determine and evaluate its health hazard. Using standard testing guidelines, tests included acute (4-h) and 4-week (6 h/day, 5 days/week) inhalation (nose-only) toxicity studies in rats, acute (10-min) inhalation cardiac sensitization study in dogs, in vitro chromosomal aberrations experiments in human lymphocytes, and in vitro mutagenic experiments in Salmonella typhimurium and Escherichia coli. There were no mortalities in rats (n = 10) exposed for 4 h to 10,000 ppm PFBI, but all rats (n = 10) died within 2 h when exposed to 20,000 ppm PFBI.[2]
The 4-h LC50 (95% confidence limits) was 14,000 ppm (13,000 ppm to 16,000 ppm). Signs (nasal discharge and labored breathing) observed in the rats exposed to 10,000 ppm returned to normal within 48 h. Perfluorobutyl iodide has the potential to cause cardiac sensitization in epinephrine-challenged dogs at 6200 ppm. A concentration of 3900 ppm was a no-observed-adverse-effect level (NOAEL) in the cardiac sensitization study. In the 4-week inhalation study (5 rats/sex/group), respiratory mucosal hypertrophy/hyperplasia was observed in rats of the 10,000-ppm group. A NOAEL of 1000 ppm was selected for the 4-week study on the basis that the mild increase in T4 observed at 1000 ppm was considered adaptive, not adverse, because of the absence of frank effects in the thyroid. In the in vitro studies, PFBI showed no evidence of either mutagenic or clastogenic activity. The toxicity profile of PFBI was compared to trifluoroiodomethane. In conclusion, the results of these studies indicate a low order of general toxicity and an absence of genotoxicity following Perfluorobutyl iodide exposure.
References
[1]Wang Y, Cao Z, He Q, Huang X, Liu J, Neumann H, Chen G, Beller M. Activation of perfluoroalkyl iodides by anions: extending the scope of halogen bond activation to C(sp3)-H amidation, C(sp2)-H iodination, and perfluoroalkylation reactions. Chem Sci. 2023 Jan 24;14(7):1732-1741. doi: 10.1039/d2sc06145g. PMID: 36819859; PMCID: PMC9930934.
[2]Dodd, Darol et al. “Perfluoro-n-butyl iodide: acute toxicity, subchronic toxicity and genotoxicity evaluations.” International journal of toxicology vol. 23,4 (2004): 249-58. doi:10.1080/10915810490502050
You may like
Lastest Price from Perfluorobutyl iodide manufacturers
US $0.00-0.00/KG2025-12-01
- CAS:
- 423-39-2
- Min. Order:
- 1KG
- Purity:
- 98
- Supply Ability:
- 10000KGS

US $0.00/kg2025-04-10
- CAS:
- 423-39-2
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 20 tons