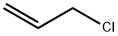
Аллил хлористый
- английское имяAllyl chloride
- CAS №107-05-1
- CBNumberCB9852695
- ФормулаC3H5Cl
- мольный вес76.52
- EINECS203-457-6
- номер MDLMFCD00000984
- файл Mol107-05-1.mol
| Температура плавления | -136 °C |
| Температура кипения | 44-46 °C(lit.) |
| плотность | 0.939 g/mL at 25 °C(lit.) |
| плотность пара | 2.6 (vs air) |
| давление пара | 20.58 psi ( 55 °C) |
| показатель преломления | n |
| Fp | −20 °F |
| температура хранения | Store at +2°C to +8°C. |
| растворимость | alcohol: miscible(lit.) |
| форма | Powder/Solid |
| цвет | White |
| Удельный вес | 0.939 |
| Запах | pungent odor |
| Пределы взрываемости | 3.3-11.2%(V) |
| Растворимость в воде | 3.6 G/L (20 ºC) |
| Точка замерзания | -134.5℃ |
| Мерк | 14,289 |
| БРН | 635704 |
| констант закона Генри | 2.69 at 25 °C (static headspace-GC, Welke et al., 1998) |
| Пределы воздействия | NIOSH REL: TWA 1 ppm (3 mg/m3), STEL 2 ppm (6 mg/m3), IDLH 250 ppm; OSHA PEL: TWA 1 ppm; ACGIH TLV: STEL 2 ppm. |
| Диэлектрическая постоянная | 8.2(20℃) |
| Стабильность | Stable, but reacts vigorously or violently with a wide variety of materials. Highly flammable. Incompatible with strong oxidizing agents, acids, amines, peroxides, chlorides of iron and aluminium, BF3, aromatic hydrocarbons, Lewis acids, metals, caustics, ammonia, ferric chloride, ethylene imine, ethylenediamine. Heat and light sensitive |
| LogP | 2.1 at 25℃ |
| Справочник по базе данных CAS | 107-05-1(CAS DataBase Reference) |
| Рейтинг продуктов питания EWG | 5 |
| FDA UNII | V2RFT0R50S |
| Справочник по химии NIST | 1-Propene, 3-chloro-(107-05-1) |
| МАИР | 3 (Vol. 36, Sup 7, 71, 125) In prep. |
| Система регистрации веществ EPA | Allyl chloride (107-05-1) |
| UNSPSC Code | 12352101 |
| NACRES | NA.22 |
| Коды опасности | F,Xn,N,T | |||||||||
| Заявления о рисках | 45-46-11-20/21/22-36/37/38-48/20-50-68-40-39/23/24/25-23/24/25-48/23/24/25 | |||||||||
| Заявления о безопасности | 53-26-36/37-45-61-46-25-16-7 | |||||||||
| РИДАДР | UN 1100 3/PG 1 | |||||||||
| OEB | B | |||||||||
| OEL | TWA: 1 ppm (3 mg/m3), STEL: 2 ppm (6 mg/m3) | |||||||||
| WGK Германия | 2 | |||||||||
| RTECS | UC7350000 | |||||||||
| F | 19 | |||||||||
| Температура самовоспламенения | 390 °C | |||||||||
| Примечание об опасности | Flammable | |||||||||
| TSCA | Yes | |||||||||
| кода HS | 2903 29 00 | |||||||||
| Класс опасности | 3 | |||||||||
| Группа упаковки | I | |||||||||
| Банк данных об опасных веществах | 107-05-1(Hazardous Substances Data) | |||||||||
| Токсичность | LD50 orally in rats: 0.7 g/kg (Smyth, Carpenter) | |||||||||
| ИДЛА | 250 ppm | |||||||||
| NFPA 704: |
|
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)




-
сигнальный язык
опасность
-
вредная бумага
H315:При попадании на кожу вызывает раздражение.
H319:При попадании в глаза вызывает выраженное раздражение.
H341:Предполагается, что данное вещество вызывает генетические дефекты.
H372:Поражает органы в результате многократного или продолжительного воздействия.
H400:Чрезвычайно токсично для водных организмов.
H351:Предполагается, что данное вещество вызывает раковые заболевания.
H225:Легковоспламеняющаяся жидкость. Пары образуют с воздухом взрывоопасные смеси.
H302+H312+H332:Вредно при проглатывании, при попадании на кожу или при вдыхании.
H371:Может поражать органы (Глаза) в результате однократного воздействия.
-
оператор предупредительных мер
P210:Беречь от тепла, горячих поверхностей, искр, открытого огня и других источников воспламенения. Не курить.
P273:Избегать попадания в окружающую среду.
P280:Использовать перчатки/ средства защиты глаз/ лица.
P303+P361+P353:ПРИ ПОПАДАНИИ НА КОЖУ (или волосы): Снять/удалить немедленно всю загрязненную одежду. Промыть кожу водой.
P304+P340+P312:ПРИ ВДЫХАНИИ: Свежий воздух, покой. Обратиться за медицинской помощью при плохом самочувствии.
P308+P311:ПРИ подозрении на возможность воздействия обратиться за медицинской помощью.
Аллил хлористый химические свойства, назначение, производство
Химические свойства
Allyl chloride is a colorless liquid, insoluble in water but soluble in common organic solvents. Allyl chloride is prepared by the reaction of propylene with chlorine. It is a common alkylating agent relevant to the manufacture of pharmaceuticals and pesticides. It is also a component in some thermo-setting resins. Allyl chloride has been produced commercially since 1945 and is used almost exclusively as a chemical intermediate, principally in the production of epichlorohydrin or as a raw material for epichlorohydrin. It is also used as a chemical intermediate in the preparation of glycerin, glycerol chlorohydrins, glycidyl ethers, allylamines, and allyl ethers of trimethylpropane, sodium allyl sulfonate, a series of allyl amines and quaternary ammonium salts, allyl ethers, and a variety of alcohols, phenols, and polyols. It is also used in pharmaceuticals as a raw material for the production of allyl isothiocyanate (synthetic mustard oil), allyl substituted barbiturates (sedatives), and cyclopropane (anesthetic); in the manufacture of specialty resins for water treatment and to produce babiturate and hypnotic agents such as aprobarbital, butalbital, methohexital sodium, secobarbital, talbutal, and thiamyl sodium.Физические свойства
Colorless to light brown to reddish-brown liquid with a pungent, unpleasant, garlic-like odor. An experimentally determined odor threshold concentration of 470 ppbv was reported by Leonardos et al. (1969).История
Allyl chloride, the only chloropropene of industrial importance, was first produced in 1857 by A. CAHOURS and A. W. HOFMANN by reacting phosphorus chloride with allyl alcohol. The name allyl is derived from the latin allium, meaning garlic. Inhalation of even small amounts of allyl chloride produces, after a short time, the characteristic odor of garlic on the breath. At the end of the 1930s, IG Farbenindustrie and the Shell Development Co. developed the high-temperature chlorination of propene, permitting large-scale production of allyl chloride with good yields. A significant part of the development was done by the Shell Chemical Co. when erecting a commercial plant in 1945. Dow, Solvay, and Asahi-Kashima developed their own processes.Использование
Allyl chloride (3-chloropropene; 1-chloro-2-propene) is a chemical intermediate used in the synthesis of allyl compounds found in varnish, resins, polymers, pesticides, and pharmaceuticals (O’Neil, 2001).Методы производства
Allyl chloride can be synthesized by reaction of allyl alcohol with HCl or by treatment of allyl formate with HCl in the presence of a catalyst (ZnCl2).Общее описание
A clear colorless liquid with an unpleasant pungent odor. Flash point -20°F. Boiling point 113°F. Less dense than water (7.8 lb / gal) and insoluble in water. Hence floats on water. Vapor irritates skin, eyes and mucous membranes. Vapors are heavier than air. Long exposure to low concentrations or short exposure to high concentrations may have adverse health effects from inhalation or skin absorption.Реакции воздуха и воды
Highly flammable. Insoluble in water.Профиль реактивности
Allyl chloride presents a serious fire and explosion hazard when exposed to heat, flame or oxidizing agents. Polymerizes violently and exothermically with Lewis acids (aluminum chloride, boron trifluoride, sulfuric acid) or metals (aluminum, magnesium, zinc, or galvanized metal) [MCA SD-99, 1973]. Incompatible with acids (nitric acid, chlorosulfonic acid, oleum), with strong bases (sodium hydroxide, potassium hydroxide), with ethyleneimine and ethylenediamine [Lewis, 3rd ed., 1993, p. 36]. Attempts to alkylate benzene or toluene using Allyl chloride in the presence of ethylaluminum chlorides have led to explosions.Опасность
Skin and eye irritant. Upper respiratory tract irritant, liver and kidney damage. Question- able carcinogen.Угроза здоровью
Allyl chloride is toxic and flammable. Exposures to allyl chloride cause a cough, sore throat, headache, dizziness, weakness, respiratory distress, abdominal pain, burning sensation, vomiting, and loss of consciousness. After acute inhalation exposures to high levels of allyl chloride, workers developed irritation of the eyes and respiratory passages, loss of consciousness, and fatal injury. Prolonged and intense exposure produced conjunctivitis, reddening of eyelids, and corneal burn, damage to the CNS, causing motor and sensory neurotoxic damage, and the heart and respiratory system, causing the onset of pulmonary edema in humans. Laboratory rabbits exposed to allyl chloride through inhalation developed degenerative changes that included dilation of sinusoids and vacuolar degeneration in the liver, congestion or cloudy swelling and fatty degeneration of the epithelium of the renal convoluted tubules, and thickening of the alveolar septa in the lungs. The exposed cat exhibited only muscle weakness and unsteady gait toward the end of the exposure period.Пожароопасность
Special Hazards of Combustion Products: Releases irritating hydrogen chloride gas on combustionТоксикология
Acute and Subacute Toxicity: LD50=460 mg/kg (rat, oral); LD50= 3.7 mg/kg (rabbit, percutaneous); LC50= 11 mg/L (rat, inhalation, 2 h). The inhalation of 3 ppm allyl chloride during 7 h/d on 5 days a week was tolerated by a group of rats, guinea pigs, and rabbits for 180 days without irreversible damage occurring. An analogous test using 8 ppm over a period of 35 days led to damage of the liver and kidneys. Further experiments demonstrate a neurotoxic effect of allyl chloride, in particular to the peripheral nerves of cats and rabbits.Возможный контакт
Allyl chloride is used as a chemical intermediate and in making allyl compounds, epichlorohydrin, and glycerol.Канцерогенность
The IARC found that it could not classify AC as a human carcinogen on the basis of available data. In contrast, EPA considers AC to be a possible human carcinogen and has ranked it in EPA’s Group C. This classification was based on a low incidence of forestomach tumors in female mice and positive results in a variety of genetic toxicity tests. However, the forestomach tumor data were not used for quantitative cancer risk assessment. AC is a strong alkylating agent and is structurally similar to other forestomach carcinogens, such as propylene oxide and epichlorohydrin, which cause tumors at the site of exposure.Olsen reported on a cohort of 1064 men employed at a Texas plant in epoxy resin, glycerin, andAC/epichlorohydrin production between 1957 and 1986 and followed up through 1989. There were 66 total deaths [standardized mortality ratio (SMR)=0.8; 95% CI 0.6–1.0] and 10 cancers (SMR=0.5; CI 0.2–0.9).However, the authors noted that the cohort was limited due to sample size, duration of follow-up, small numbers of deaths both expected and found, and the limited exposure potential.
Экологическая судьба
Biological. Bridié et al. (1979) reported BOD and COD values of 0.23 and 0.86 g/g using filtered effluent from a biological sanitary waste treatment plant. These values were determined using a standard dilution method at 20 °C and stirred for a period of 5 d. When a sewage seed was used in a separate screening test, a BOD value of 0.42 g/g was obtained. The ThOD for allyl chloride is 1.67 g/g.Photolytic. Anticipated products from the reaction of allyl chloride with ozone or OH radicals in the atmosphere are formaldehyde, formic acid, chloroacetaldehyde, chloroacetic acid, and chlorinated hydroxy carbonyls (Cupitt, 1980).
Chemical/Physical. Hydrolysis under alkaline conditions will yield allyl alcohol (Hawley, 1981). The estimated hydrolysis half-life in water at 25 °C and pH 7 is 2.0 yr (Mabey and Mill, 1978).
Перевозки
UN1100 Allyl chloride, Hazard Class: 3; Labels: 3-Flammable liquid, 6.1-Poisonous materialsМетоды очистки
Likely impurities include 2-chloropropene, propyl chloride, iso-propyl chloride, 3,3-dichloropropane, 1,2-dichloropropane and 1,3-dichloropropane. Purify it by washing with conc HCl, then with Na2CO3 solution, dry it with CaCl2, and distil it through an efficient column [Oae & Vanderwerf J Am Chem Soc 75 2724 1953]. [Beilstein 1 IV 738.] LACHRYMATORY, TOXIC.Несовместимости
Contact with water forms hydrochloric acid. Keep away from strong oxidizers, acids, aluminum, amines, peroxides, chlorides of iron and aluminum; magnesium, zinc.Утилизация отходов
Consult with environmental regulatory agencies for guidance on acceptable disposal practices. Generators of waste containing this contaminant (≥100 kg/mo) must conform with EPA regulations governing storage, transportation, treatment, and waste disposal. Controlled incineration at a temperature of 982 C for 2 seconds minimum.Аллил хлористый запасные части и сырье
сырьё
1of3
запасной предмет
- Этил 4-ацетилбензоа
- Диаллилфталатная
- 3-Acetylbenzonitrile
- Molosultap
- [[о-(аллилокси)фенокси]метил]оксиран
- 2- (1-гидрокси-3-бутенил) -5-метилфуран
- 3-(2-BROMOACETYL)BENZOIC ACID
- 1, 2-ДИБРОМ-3-ХЛОРПРОПАН
- 2-Allyl-4-hydroxy-3-methyl-2-cyclopenten-1-one
- Fluorochloridone
- 4-Phenyl-1-butene
- N,N-диметилаллиламин
- 3-(2-метил-1,3-тиазол-4-ил)бензойная кислота
- 1,3-ДИХЛОРО-2-ПРОПАНОЛ
- 3-ацетилбензойной кислоты
1of8
Аллил хлористый поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия | |
|---|---|---|---|---|---|
| +8618601192704 | China | 76 | 58 | ||
| +86-0543-2240078 +8618364991597 |
China | 994 | 58 | ||
| +8615531157085 | China | 8804 | 58 | ||
| +86 13288715578 +8613288715578 |
China | 12825 | 58 | ||
| +8617531153977 | China | 5855 | 58 | ||
| +86-13131129325 | China | 5887 | 58 | ||
| +86-371-86557731 +86-13613820652 |
China | 20259 | 58 | ||
| +86-(0)57185586718 +86-13336195806 |
China | 29792 | 60 | ||
| +86-0371-55170693 +86-19937530512 |
China | 21632 | 55 | ||
| +86-0551-65418679 +8618949832763 |
China | 2986 | 55 |
Аллил хлористый Обзор)
- Диэтиловый ди-н-propylmalonate
- Диэтил diallylmalonate
- 1-хлорпропан
- Малоновая кислота
- ГЕСАХЛОРОЦИКЛОПЕНТАДИЕН
- 2,3-дихлор-1-пропена
- Изопропил хлористый
- Аллилпалладия (II) хлорид димер
- Аллил хлористый
- 3-Хлор-2-метилпропена
1of4