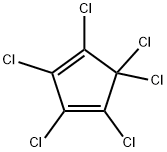
ГЕСАХЛОРОЦИКЛОПЕНТАДИЕН
- английское имяHexachlorocyclopentadiene
- CAS №77-47-4
- CBNumberCB3432075
- ФормулаC5Cl6
- мольный вес272.77
- EINECS201-029-3
- номер MDLMFCD00001352
- файл Mol77-47-4.mol
| Температура плавления | −10 °C(lit.) |
| Температура кипения | 239 °C753 mm Hg(lit.) |
| плотность | 1.702 g/mL at 25 °C(lit.) |
| давление пара | 0.13 psi ( 20 °C) |
| показатель преломления | n |
| Fp | 109 °C |
| температура хранения | 2-8°C |
| растворимость | Chloroform (Sparingly), Ethyl Acetate (Slightly) |
| форма | Yellow to amber-colored liquid |
| цвет | Colourless to Pale Yellow |
| Растворимость в воде | 805ug/L(22.5 ºC) |
| констант закона Генри | 1.64(x 10-2 atm?m3/mol) at 25 °C (gas stripping-GC, Warner et al., 1987) 1.6(x 10-2 atm?m3/mol) (Pankow and Rosen, 1988) |
| Пределы воздействия | NIOSH REL: 10 ppb (100 mg/m3); ACGIH TLV: TWA 0.01 ppm (adopted), 0.002 mg/m3 ppm (skin). |
| Диэлектрическая постоянная | 2.9100000000000001 |
| Стабильность | Stability Stable, but light-sensitive. Non-flammable. Very reactive with alkenes and polynuclear hydrocarbons. Explosive with sodium. Incompatible with strong oxidizing agents, most common metals. |
| Стандарт первичной питьевой воды EPA | MCL:0.05,MCLG:0.05 |
| Справочник по базе данных CAS | 77-47-4(CAS DataBase Reference) |
| Рейтинг продуктов питания EWG | 4 |
| FDA UNII | IP6ATU242I |
| Справочник по химии NIST | 1,3-Cyclopentadiene, 1,2,3,4,5,5-hexachloro-(77-47-4) |
| Система регистрации веществ EPA | Hexachlorocyclopentadiene (77-47-4) |
| Коды опасности | T+,N,T,F |
| Заявления о рисках | 22-24-26-34-50/53-39/23/24/25-23/24/25-52/53-11 |
| Заявления о безопасности | 23-39-45-61-60-53-25-36/37-16 |
| РИДАДР | UN 2646 6.1/PG 1 |
| OEB | D |
| OEL | TWA: 0.01 ppm (0.1 mg/m3) |
| WGK Германия | 3 |
| RTECS | GY1225000 |
| F | 10-23 |
| Класс опасности | 6.1(a) |
| Группа упаковки | I |
| Банк данных об опасных веществах | 77-47-4(Hazardous Substances Data) |
| Токсичность | Drinking water standard (final): MCLG: 50 μg/L:MCL: 50 μg/L. In addition, a DWEL of 200 μg/L was recommended (U.S. EPA, 2000). |
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)



-
сигнальный язык
опасность
-
вредная бумага
H302:Вредно при проглатывании.
H314:При попадании на кожу и в глаза вызывает химические ожоги.
H410:Чрезвычайно токсично для водных организмов с долгосрочными последствиями.
H330:Смертельно при вдыхании.
H311:Токсично при попадании на кожу.
-
оператор предупредительных мер
P260:Не вдыхать газ/ пары/ пыль/ аэрозоли/ дым/ туман.
P273:Избегать попадания в окружающую среду.
P280:Использовать перчатки/ средства защиты глаз/ лица.
P284:Использовать средства защиты органовдыхания.
P305+P351+P338:ПРИ ПОПАДАНИИ В ГЛАЗА: Осторожно промыть глаза водой в течение нескольких минут. Снять контактные линзы, если Вы ими пользуетесь и если это легко сделать. Продолжить промывание глаз.
P310:Немедленно обратиться за медицинской помощью.
ГЕСАХЛОРОЦИКЛОПЕНТАДИЕН химические свойства, назначение, производство
Описание
Hexachlorocyclopentadiene is a pale-yellow/lemon-yellow liquid with a characteristic musty or pungent odour (odour threshold – 0.03 ppm). Hexachlorocyclopentadiene does not occur naturally but is a manufactured chemical. It easily evaporates into the air. Hexachlorocyclopentadiene is the key intermediate in the manufacture of some pesticides, including heptachlor, chlordane, aldrin, dieldrin, and endrin. Hexachlorocyclopentadiene is also used in the manufacture of flame retardants and some resins, shock-proof plastics, fluorocarbons, and dyes. Hexachlorocyclopentadiene quickly breaks down by sunlight and reacts with other chemicals in the air.Химические свойства
Hexachlorocyclopentadiene is a pale-yellow to amber-colored, oily liquid. Pungent, unpleasant odor. The odor threshold is 0.15 0.33 ppm. Insoluble in water, soluble in ether, carbon tetrachloride and other organic solvents.Использование
Hexachlorocyclopentadiene is an organochlorine compound that is used to manufacturing organochlorine insecticides and acaricides, such as mirex, aldrin, dieldrin, chlordane, endosulfan, etc. It is also used to make flame retardants, resins that won't burn, shock-proof plastics, esters, ketones, fluorocarbons, and dyes.Подготовка
Hexachlorocyclopentadiene is obtained by chlorination of cyclopentadiene in two steps. In addition, using petroleum pentane as raw material, hexachlorocyclopentadiene is synthesized by photochlorination and high temperature chlorination, and the yield is about 70%.прикладной
Hexachlorocyclopentadiene(Hex) is an Intermediate in the manufacture of chlorinated pesticides and flame retardants. The principal end use for Hex is as a key intermediate in the production of chlorinated cyclodiene pesticides, including aldrin, dieldrin, endrin, chlordane, heptachlor, kepone, endosulfan, pentac, isodrin, and mirex. It is also used as an intermediate in the manufacture of flame retardants such as Dechlorane Plus and chlorendic anhydride and, to a lesser extent, in the manufacture of nonflammable resins, polyester resins, pharmaceuticals, unbreakable plastics, acids, esters, ketones, fluorocarbons, and dyes.Общее описание
A pale yellow liquid with a pungent odor. Density 14.3 lb /gal. Solidifies at 50°F. Insoluble in water. Noncombustible. Very toxic by skin absorption and inhalation. Corrosive to tissue.Реакции воздуха и воды
Insoluble in water. Reacts slowly with water to form hydrochloric acid.Профиль реактивности
Hexachlorocyclopentadiene is incompatible with strong oxidizing and reducing agents. Also incompatible with many amines, nitrides, azo/diazo compounds, alkali metals (sodium), and epoxides.Угроза здоровью
Hexachlorocyclopentadiene is very toxic and may be fatal if inhaled, swallowed, or absorbed through the skin. The probable human lethal dose is 50-500 mg/kg, or between 1 teaspoon and 1 ounce for a 150 lb. (70 kg) person. Severe exposure induces pulmonary hyperemia and edema, degenerative and necrotic changes in brain, heart and adrenal glands and necrosis of liver and kidney tubules. Questionable carcinogen.Пожароопасность
Toxic hydrogen chloride, chlorine, and phosgene gases may form in fires. In presence of moisture, will corrode iron and other materials; flammable and explosive hydrogen gas may collect in enclosed space. Will corrode iron and other metals in the presence of moisture. Reacts slowly with water to form hydrochloric acid; however, the reaction is not hazardous. Hazardous polymerization may not occur.Безопасность
Hexachlorocyclopentadiene is a manufactured chemical that does not occur naturally. Hex has no end uses of its own, and is very toxic following acute (short-term) oral and inhalation exposures. It easily evaporates into the air; the vapor looks like a blue haze. Most of the Hexachlorocyclopentadiene in the environment results from its release during production and disposal. Animal tests suggest that very high levels can cause death. Human data are limited, but it can cause headaches and irritate the nose, throat, eye, and skin.Возможный контакт
Hexachlorocyclopentadiene is used to produce the flame retardant chlorendic anhydride, which has applications in polyesters; and to produce chlorendic anhydride and chlorendic acid; which is used as a flame retardant in resins. Hexachlorocyclopentadiene is also used as an intermediate in the production of pesticides, such as aldrin, dieldrin, and endosulfan.Перевозки
UN2646 Hexachlorocyclopentadiene, Hazard Class: 6.1; Labels: 6.1-Poison Inhalation Hazard, Inhalation Hazard Zone B.Методы очистки
Dry the diene with MgSO4, filter, and distil it under vacuum in a nitrogen atmosphere. Irritates skin and eyes, HIGHLY TOXIC. [McBee et al. J Am Chem Soc 77 4378 1955, UV spectra: Idol et al. J Org Chem 20 1746 1955, Beilstein 5 III 308, 5 IV 381.]Несовместимости
Reacts slowly with water to form hydro chloric acid; will corrode iron and most metals in presence of moisture. Explosive hydrogen gas may collect in enclosed spaces in the presence of moisture. Contact with sodium may be explosive.Утилизация отходов
Incineration after mixing with another combustible fuel. Care must be exercised to assure complete combustion to prevent the formation of phosgene. An acid scrubber is necessary to remove the halo acids pro duced. Consult with environmental regulatory agencies for guidance on acceptable disposal practices. Generators of waste containing this contaminant (≥100 kg/mo) must con form to EPA regulations governing storage, transportation, treatment, and waste disposal.