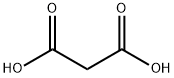
Малоновая кислота
- английское имяMalonic acid
- CAS №141-82-2
- CBNumberCB9709256
- ФормулаC3H4O4
- мольный вес104.06
- EINECS205-503-0
- номер MDLMFCD00002707
- файл Mol141-82-2.mol
химическое свойство
| Температура плавления | 132-135 °C (dec.) (lit.) |
| Температура кипения | 140℃(decomposition) |
| плотность | 1.619 g/cm3 at 25 °C |
| давление пара | 0-0.2Pa at 25℃ |
| показатель преломления | 1.4780 |
| Fp | 157°C |
| температура хранения | Sealed in dry,Room Temperature |
| растворимость | 1 M NaOH: soluble100mg/mL, clear to slightly hazy, colorless to faintly yellow |
| форма | Liquid |
| пка | 2.83(at 25℃) |
| цвет | White |
| РН | 3.17(1 mM solution);2.5(10 mM solution);1.94(100 mM solution) |
| Растворимость в воде | 1400 g/L (20 ºC) |
| Мерк | 14,5710 |
| БРН | 1751370 |
| Стабильность | Stable. Incompatible with oxidizing agents, reducing agents, bases. |
| ИнЧИКей | OFOBLEOULBTSOW-UHFFFAOYSA-N |
| LogP | -0.81 |
| Справочник по базе данных CAS | 141-82-2(CAS DataBase Reference) |
| Рейтинг продуктов питания EWG | 1 |
| FDA UNII | 9KX7ZMG0MK |
| Справочник по химии NIST | Malonic acid(141-82-2) |
| Система регистрации веществ EPA | Propanedioic acid (141-82-2) |
| UNSPSC Code | 85151701 |
| NACRES | NA.24 |
больше
| Коды опасности | Xn,Xi | |||||||||
| Заявления о рисках | 20/22-41-36/37/38-22 | |||||||||
| Заявления о безопасности | 26-36/39-37/39-36 | |||||||||
| РИДАДР | 3261 | |||||||||
| WGK Германия | 1 | |||||||||
| RTECS | OO0175000 | |||||||||
| TSCA | Yes | |||||||||
| Группа упаковки | III | |||||||||
| кода HS | 29171910 | |||||||||
| Банк данных об опасных веществах | 141-82-2(Hazardous Substances Data) | |||||||||
| Токсичность | mouse,LD50,intraperitoneal,300mg/kg (300mg/kg),National Technical Information Service. Vol. AD277-689, | |||||||||
| NFPA 704: |
|
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)

-
сигнальный язык
опасность
-
вредная бумага
H318:При попадании в глаза вызывает необратимые последствия.
-
оператор предупредительных мер
P280:Использовать перчатки/ средства защиты глаз/ лица.
P305+P351+P338:ПРИ ПОПАДАНИИ В ГЛАЗА: Осторожно промыть глаза водой в течение нескольких минут. Снять контактные линзы, если Вы ими пользуетесь и если это легко сделать. Продолжить промывание глаз.
Малоновая кислота химические свойства, назначение, производство
Описание
Malonic acid (MA), also known as propanedioic acid, is a dicarboxylic acid with structure CH2(COOH)2. It have three kinds of crystal forms, of which two are triclinic, and one is monoclinic. That crystallized from ethanol is white triclinic crystals.It decomposes to acetic acid and carbon dioxide at 140℃. It does not decompose at 1.067×103~1.333×103Pa vacuum, but directly sublimates. The ionised form of malonic acid, as well as its esters and salts, are known as malonates. For example, diethyl malonate is malonic acid's ethyl ester. The name originates from Latin malum, meaning apple.Химические свойства
Malonic acid is a white crystalline solid that decomposes at approximately 135°C. It has high solubility in water and oxygenated solvents and exhibits greater acidity than acetic acid, which has a pK value of 4.75. The pKa values for the loss of its first and second protons are 2.83 and 5.69, respectively. It is slightly soluble in pyridine. It can decompose to formic acid and carbon dioxide in case of potassium permanganate. Since that malonic acid generates carbon dioxide and water after heated without pollution problems, it can be directly used as aluminum surface treatment agent.Использование
Malonic acid is used as an intermediate in the manufacture of barbiturates and other pharmaceuticals. It is a component used as a stabilizer in many high-end cosmetic and pharmaceutical products. Malonic acid is also used as building block in chemical synthesis, specifically to introduce the molecular group -CH2-COOH. It is used for the introduction of an acetic acid moiety under mild conditions by Knoevenagel condensation and subsequent decarboxylation.прикладной
Malonic acid is acts as a building block in organic synthesis. It is also useful as a precursor for polyesters and alkyd resins, which is used in coating applications, thereby protecting against UV light, corrosion and oxidation. It acts as a cross linker in the coating industry and surgical adhesive. It finds application in the production of specialty chemicals, flavors and fragrances, polymer cross linkers and pharmaceuticals.Определение
ChEBI: Malonic acid is an alpha,omega-dicarboxylic acid in which the two carboxy groups are separated by a single methylene group. It has a role as a human metabolite. It is a conjugate acid of a malonate(1-).Подготовка
Malonic acid is usually produced from chloroacetic acid.Reaction: The chloroacetic acid is added to the reaction kettle by adding sodium carbonate aqueous solution to generate sodium chloroacetate aqueous solution, and then 30% sodium cyanide solution is slowly added dropwise, and the reaction is carried out at a predetermined temperature to generate sodium cyanoacetate. After the cyanation reaction is completed, add sodium hydroxide for heating and hydrolysis to generate sodium malonate solution, concentrate, then dropwise add sulfuric acid for acidification to generate malonic acid, filter and dry to obtain the product.
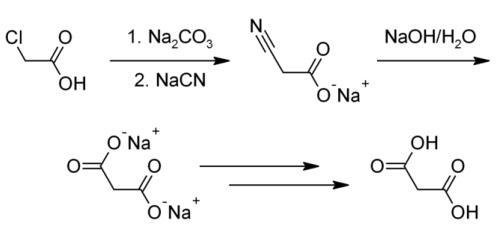
This method often does not produce a pure enough product or the pure product has an extremely low yield. Industrially, malonic acid is also produced by hydrolyzing dimethyl malonate or diethyl malonate. This manufacturing method is able to bring about a higher yield and purity, but the organic synthesis of malonic acid through these processes is extremely costly and environmentally hazardous.
Реакции
In a well - known reaction, malonic acid condenses with urea to form barbituric acid. Malonic acid is also frequently used as an enolate in Knoevenagel condensations or condensed with acetone to form Meldrum's acid. The esters of malonic acid are also used as a - CH2COOH synthon in the malonic ester synthesis.Биологические функции
Malonic acid is the classic example of a competitive inhibitor of the enzyme succinate dehydrogenase (complex II), in the respiratory electron transport chain.It binds to the active site of the enzyme without reacting, competing with the usual substrate succinate but lacking the ?CH2CH2? group required for dehydrogenation. This observation was used to deduce the structure of the active site in succinate dehydrogenase.Общее описание
White crystals or crystalline powder. Sublimes in vacuum.Реакции воздуха и воды
Water soluble.Опасность
Strong irritant.Пожароопасность
Flash point data for Malonic acid are not available; however, Malonic acid is probably combustible.Приложение биотехнологий?
The calcium salt of malonic acid occurs in high concentrations in beetroot. It exists in its normal state as white crystals. Malonic acid is the classic example of a competitive inhibitor: It acts against succinate dehydrogenase (complex II) in the respiratory electron transport chain.Методы очистки
Crystallise malonic acid from *benzene/diethyl ether (1:1) containing 5% of pet ether (b 60-80o), wash with diethyl ether, then recrystallise it from H2O or acetone. Dry it under vacuum over conc H2SO4. [Beilstein 2 IV 1874.]Малоновая кислота запасные части и сырье
сырьё
1of2
запасной предмет
- 4-(МОРФОЛИН-4-ИЛМЕТИЛ)-1,3-ТИАЗОЛ-2-АМИН
- Диэтил кетомалона
- 3 - (3,4,5-триметоксифенил) пропионовой кислоты
- Тартроновая кислота
- Methyl trans-2-nonenoate
- trans-2,4,5-Trimethoxycinnamic acid
- 4-ХЛОРО-БЕТА-МЕТИЛ-Y-ОКСО-БЕНЗОЛБУТАНОВАЯ КИСЛОТА
- 5-гексилдигидро-2 (3H) -фуранон
- Димедон
- 2 - (3-пиридил) пропионовой кислоты
- trans-Ferulic acid
- транс-3-Гексеновая кислота
- 4-БРОМ-ПИРАН-2-ОДИН
- 4-гидрокси-7-метил-1,8-нафтиридин-3-карбоновая кислота
- 6-METHOXY-2-OXO-2H-CHROMENE-3-CARBOXYLIC ACID
- 3 - (трифторметокси) коричной кислоты
- Диэтиловый н-бутилмалона
- 3,3-DIMETHOXYESTR-5(10)-ENE-17 B OL
- 3-АМИНО-3-(2-ТИЕНИЛ)ПРОПАНОВАЯ КИСЛОТА
- Диэтил (фенилацетил) малонат
- Antifreeze
- Диэтиловый втор-бутилмалона
- 4-Ethoxycinnamic кислота
- 3,5-DIMETHOXYCINNAMIC ACID
- (1H-ИНДАЗОЛ-3-YL) -УКСУСНАЯ КИСЛОТА
- 4-фторкоричную кислота
- 3 - (2-метилфенил) пропионовой кислоты
- 5-BROMO-2-FLUOROCINNAMIC ACID
- 3 - (3-хлорфенил) пропионовой кислоты
- 3 - (4-метилфенил) пропионовой кислоты
1of8
Малоновая кислота поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия | |
|---|---|---|---|---|---|
| +86-15350851019; +8615350851019 |
China | 1000 | 58 | ||
| +86-021-57951555 +8617317452075 |
China | 1803 | 55 | ||
| +86-0551-65418671 +8618949823763 |
China | 34563 | 58 | ||
| +86-027-85615902 +86-13971435335 |
China | 29 | 58 | ||
| +86-371-86557731 +86-13613820652 |
China | 20202 | 58 | ||
| +8615637360870 | China | 12 | 58 | ||
| +8617732866630 | China | 8773 | 58 | ||
| +86-29-89586680 +86-15129568250 |
China | 21550 | 58 | ||
| +86-13131129325 | China | 5251 | 58 | ||
| +8613343047651 | China | 3692 | 58 |