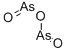
Оксид мышьяка (III)
- английское имяArsenic(III) oxide
- CAS №1327-53-3
- CBNumberCB9236345
- ФормулаAs2O3
- мольный вес197.84
- EINECS215-481-4
- номер MDLMFCD00003433
- файл Mol1327-53-3.mol
| Температура плавления | 312.3 °C | ||||||||||||||
| Температура кипения | 465°C | ||||||||||||||
| плотность | 3,738 g/cm3 | ||||||||||||||
| давление пара | 0.033Pa at 25℃ | ||||||||||||||
| Fp | 465°C subl. | ||||||||||||||
| температура хранения | Poison room | ||||||||||||||
| растворимость | 37 g/L (20°C) | ||||||||||||||
| форма | Powder | ||||||||||||||
| Удельный вес | 3.738 | ||||||||||||||
| цвет | White | ||||||||||||||
| Растворимость в воде | 37 g/L (20 ºC) | ||||||||||||||
| Мерк | 14,804 | ||||||||||||||
| crystal system | Monoclinic | ||||||||||||||
| Space group | P21/n | ||||||||||||||
| Lattice constant |
|
||||||||||||||
| Справочник по базе данных CAS | 1327-53-3(CAS DataBase Reference) | ||||||||||||||
| Рейтинг продуктов питания EWG | 1 | ||||||||||||||
| Словарь онкологических терминов NCI | arsenic trioxide | ||||||||||||||
| FDA UNII | S7V92P67HO | ||||||||||||||
| Словарь наркотиков NCI | arsenic trioxide | ||||||||||||||
| Код УВД | L01XX27 | ||||||||||||||
| Справочник по химии NIST | Diarsenic oxide(1327-53-3) | ||||||||||||||
| Система регистрации веществ EPA | Arsenic(III) trioxide (1327-53-3) | ||||||||||||||
| Пестициды Закон о свободе информации (FOIA) | Arsenic trioxide | ||||||||||||||
| UNSPSC Code | 12171602 | ||||||||||||||
| NACRES | NA.55 |
| Коды опасности | T+,N | |||||||||
| Заявления о рисках | 45-28-34-50/53 | |||||||||
| Заявления о безопасности | 53-45-60-61 | |||||||||
| РИДАДР | UN 1561 6.1/PG 2 | |||||||||
| WGK Германия | 3 | |||||||||
| RTECS | CG3325000 | |||||||||
| TSCA | Yes | |||||||||
| Класс опасности | 6.1 | |||||||||
| Группа упаковки | II | |||||||||
| кода HS | 28112990 | |||||||||
| Банк данных об опасных веществах | 1327-53-3(Hazardous Substances Data) | |||||||||
| Токсичность | LD50 in mice, rats (mg/kg): 39.4, 15.1 orally (Harrison) | |||||||||
| NFPA 704: |
|
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)




-
сигнальный язык
опасность
-
вредная бумага
H318:При попадании в глаза вызывает необратимые последствия.
H350:Может вызывать раковые заболевания.
H400:Чрезвычайно токсично для водных организмов.
H314:При попадании на кожу и в глаза вызывает химические ожоги.
H410:Чрезвычайно токсично для водных организмов с долгосрочными последствиями.
H300:Смертельно при проглатывании.
-
оператор предупредительных мер
P303+P361+P353:ПРИ ПОПАДАНИИ НА КОЖУ (или волосы): Снять/удалить немедленно всю загрязненную одежду. Промыть кожу водой.
P305+P351+P338:ПРИ ПОПАДАНИИ В ГЛАЗА: Осторожно промыть глаза водой в течение нескольких минут. Снять контактные линзы, если Вы ими пользуетесь и если это легко сделать. Продолжить промывание глаз.
P405:Хранить в недоступном для посторонних месте.
Оксид мышьяка (III) химические свойства, назначение, производство
Описание
Arsenic trioxide, often denoted as As2O3 butmore correctly stated as As4O6, is an inorganic compound mainly used as the precursor for organoarsenic compounds. It can be obtained by the oxidation of arsenic-containing minerals in the air, such as roasting of orpiment.2As2S3 + 9O2 → As4O6 + 6SO2
Химические свойства
WHITE POWDER AND FINE CHUNKSИспользование
It is a reductometric standard.Определение
A colorless crystalline solid that is very poisonous (0.1 g would be a lethal dose). Analysis of the solid and vapor states suggests a dimerized structure of As4O6. An amphoteric oxide, arsenic(III) oxide is sparingly soluble in water, producing an acidic solution. It is formed when arsenic is burned in air or oxygen.Подготовка
Arsenic trioxide is obtained by roasting the mineral arsenopyrite, FeAsS, in air at 650 to 700°C. It is also obtained as a by-product during the smelting ofcopper and lead concentrates during the extraction of these metals from their ores that contain arsenic. The latter readily oxidizes to arsenic trioxide which is volatilized. The vapors are then condensed and collected. High purity-grade oxide can be obtained by resublimation of the crude trioxide or by pressure leaching and recrystallization. Arsenic trioxide may also be prepared by hydrolysis of arsenic trichloride, -tribromide or -trifluoride.Реакции
In the processing of As2O3 the oxide is normally reduced withcarbon:2As2O3+3C?→As4+3CO2
The reaction is endothermic and is carried out at 500–800℃.The elemental arsenic sublimes and is condensed out of the reaction gas by cooling.
Общее описание
White or transparent, glassy amorphous lumps or crystalline powder. Slightly soluble in water, but dissolves very slowly; more soluble in hot water. Noncombustible. Corrosive to metals in the presence of moisture. Toxic by ingestion.Реакции воздуха и воды
Slightly soluble in water, but dissolves very slowly; more soluble in hot water [Merck].Профиль реактивности
Arsenic(III) oxide reacts vigorously with fluorine at ordinary temperatures [Mellor 9:34 1946-47]. Dissolves in aqueous acids. Incompatible with tannic acid, infusions of cinchona and other vegetable astringent infusions and decoctions, and with iron in solution [Merck].Опасность
A confirmed carcinogen.Угроза здоровью
Material is considered super toxic; probable oral lethal dose (human) is less than 5 mg/kg, i.e., a taste (less than 7 drops) for a 70kg (150 lb.) person. Material causes acute gastrointestinal and central nervous system symptoms. Renal and hepatic damage have also been observed. Chronic exposure to material has led to nasal septum perforation, dermatological symptoms (lesions, necrosis, etc.) and an increase in the incidence of lung and lymphatic cancers. Appreciable exposure to respiratory irritant promoters such as metal oxide fumes elicits a carcinogenic response from Arsenic(III) oxide .Пожароопасность
Toxic fumes of Arsenic(III) oxide and arsine may be formed in fire situations. Contact with halide acids will form toxic volatile halides. Reduction in acid solutions will form arsine. Arsenic(III) oxide and excess zinc filings will explode on heating. Avoid sodium chlorate; fluorine; chlorine trifluoride; chromic oxide; aluminum chloride; phosphorus pentoxide; hydrogen fluoride; oxygen difluoride, tannic acid; infusion cinchona and other vegetable astringent infusions and decoctions; iron in solution. Arsenic(III) oxide is stable in air but slowly oxidizes in acid media.Профиль безопасности
Confirmed human carcinogen with experimental neoplastigenic and tumorigenic data. Poison by ingestion, subcutaneous, and intravenous routes. Human systemic effects by ingestion: sleep changes, muscle weakness, hypermotiltty, darrhea, cardiac arrhythmias, coma, fatty degeneration of the liver, depressed renal function tests. An experimental teratogen. Other experimental reproductive effects. Mutation data reported. Reacts vigorously with Rb2C2, CIF3, F2, Hg, OF2, NaClO3. See also ARSENIC COMPOUNDS.Возможный контакт
Arsenic trioxide, a primary raw material for other arsenic compounds, is used in manufacture of pesticides, glass, industrial chemicals, and drugs. It is an intermediate for insecticides, herbicides and fungicides. The material is used as a wood and tanning preservative and a decoloring and refining agent in glass manufacture. It is also used in pharmaceuticals and in the purification of synthetic gas.Перевозки
UN1561 Arsenic trioxide, Hazard Class: 6.1; Labels: 6.1-Poisonous materialsМетоды очистки
It crystallises in an octahedral form (common form) from H2O or from dilute HCl (1:2), and is then washed, dried and sublimed (193o/760mm). Analytical reagent grade material is suitable for use as an analytical standard after it has been dried at 105o for 1-2hours or has been left in a desiccator for several hours over conc H2SO4. Alternatively: As2O3 (15g) is dissolved by heating in a mixture of H2O (60mL) and HCl (90g, s.g. 1.1), and crystallisation occurs on cooling, accompanied by brilliant flashes of light [Bandrowski Z Phys Chem 17 234 1895]. The amorphous form is a colourless transparent glass (m 200o) which is obtained when the vapour is slowly condensed below the vaporization temperature, and should be kept in a sealed tube because it changes to the octahedral form (m 275o) in the presence of moisture. [Rushton & Daniels J Am Chem Soc 48 384 1926.] A third monoclinic form, is obtained by heating the oxide in a sealed tube at 400o (the vitreous, amorphous form remains at the bottom of the tube) with the monoclinic form subliming onto the intermediate part of the tube at 200o (m 312o), and the octahedral form deposits at the top of the tube. The transition temperature between the last two forms is ~250o. POISONOUS (particularly the vapour, handle in a ventilated fume cupboard). [Schenk in Handbook of Preparative Inorganic Chemistry (Ed. Brauer) Academic Press Vol I p 600 1963.]Несовместимости
Sodium chlorate; sodium hydroxide, sulfuric acid; fluorine; chlorine trifluoride; chromic oxide; aluminum chloride; phosphorus pentoxide; hydrogen fluoride; oxygen difluoride; tannic acid; infusion cinchona and other vegetable astringent infusions and decoctions; iron in solution. Contact with acids or acid mists releases deadly arsine gas.Утилизация отходов
Consult with environmental regulatory agencies for guidance on acceptable disposal practices. Generators of waste containing this contaminant (≥100 kg/mo) must conform with EPA regulations governing storage, transportation, treatment, and waste disposal. Dissolve in a minimum of concentrated hydrochloric acid. Dilute with water until white precipitate forms. Add HCl to dissolve. Saturate with H2S; filter and wash precipitate and return to supplier. Alternatively, precipitate with heavy metals, such as lime or ferric hydroxide in lieu of H2S. If needed, seek professional environmental engineering assistance from the United States Environmental Protection Agency Environmental Response Team at (908) 548-8730 (24-hour response line).Оксид мышьяка (III) запасные части и сырье
Оксид мышьяка (III) Обзор)
1of4