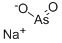
АРСЕНИТ НАТРИЯ
- английское имяSodium m-arsenite
- CAS №7784-46-5
- CBNumberCB6854335
- ФормулаAsO2.Na
- мольный вес129.91
- EINECS232-070-5
- номер MDLMFCD00085309
- файл Mol7784-46-5.mol
| Температура плавления | 817 °C(lit.) |
| Температура кипения | 613 °C(lit.) |
| плотность | 5.727 g/mL at 25 °C(lit.) |
| температура хранения | Store at +15°C to +25°C. |
| растворимость | H2O: soluble |
| форма | powder |
| цвет | off-white to gray |
| РН | 9.3 (H2O, 20°C) |
| Растворимость в воде | Miscible with water. Slightly miscible with alcohol. |
| Мерк | 13,8651 |
| Стабильность | Stable. Incompatible with strong oxidizing agents, strong acids. Protect from moisture. Absorbs carbon dioxide from air. |
| FDA UNII | 48OVY2OC72 |
| Система регистрации веществ EPA | Sodium arsenite (7784-46-5) |
| UNSPSC Code | 41116107 |
| NACRES | NA.24 |
| Коды опасности | T,N | |||||||||
| Заявления о рисках | 23/25-50/53-51/53-45-52/53-22-23/24/25-21-34 | |||||||||
| Заявления о безопасности | 20/21-28-45-60-61-53-36/37-36/37/39-26 | |||||||||
| РИДАДР | UN 1558 6.1/PG 2 | |||||||||
| WGK Германия | 3 | |||||||||
| RTECS | CG0525000 | |||||||||
| F | 10 | |||||||||
| TSCA | Yes | |||||||||
| кода HS | 2842 90 80 | |||||||||
| Класс опасности | 8 | |||||||||
| Группа упаковки | III | |||||||||
| Банк данных об опасных веществах | 7784-46-5(Hazardous Substances Data) | |||||||||
| Токсичность | LD50 orally in rats: 0.041 g/kg (Smyth) | |||||||||
| NFPA 704: |
|
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)



-
сигнальный язык
опасность
-
вредная бумага
H350:Может вызывать раковые заболевания.
H301+H331:Токсично при проглатывании или при вдыхании.
H410:Чрезвычайно токсично для водных организмов с долгосрочными последствиями.
-
оператор предупредительных мер
P201:Беречь от тепла, горячих поверхностей, искр, открытого огня и других источников воспламенения. Не курить.
P202:Перед использованием ознакомиться с инструкциями по технике безопасности.
P261:Избегать вдыхания пыли/ дыма/ газа/ тумана/ паров/ аэрозолей.
P273:Избегать попадания в окружающую среду.
P301+P310:ПРИ ПРОГЛАТЫВАНИИ: Немедленно обратиться за медицинской помощью. Прополоскать рот.
P304+P340+P311:ПРИ ВДЫХАНИИ: Свежий воздух, покой. Обратиться за медицинской помощью.
АРСЕНИТ НАТРИЯ химические свойства, назначение, производство
Химические свойства
white granular crystalsИспользование
Technical grade in manufacture of arsenical soap for use on skins, for treating vines against certain scale diseases; as insecticide especially for termites.Определение
ChEBI: An inoganic sodium salt with formula with formula NaAsO2.Общее описание
A white or grayish-white powder. Denser than water. Contact may irritate skin, eyes and mucous membranes. May be toxic by ingestion, inhalation and skin absorption.Реакции воздуха и воды
Soluble in water. Slowly converted in solution to arsenates by atmospheric oxygen; in dry state Sodium m-arsenite is decomposed by carbon dioxide. [EPA, 1998].Профиль реактивности
Salts, basic, such as SODIUM ARSENITE, are generally soluble in water. The resulting solutions contain moderate concentrations of hydroxide ions and have pH's greater than 7.0. They react as bases to neutralize acids. These neutralizations generate heat, but less or far less than is generated by neutralization of the bases in reactivity group 10 (Bases) and the neutralization of amines. They usually do not react as either oxidizing agents or reducing agents but such behavior is not impossible.Опасность
Toxic by ingestion and inhalation.Угроза здоровью
Extremely toxic: probable oral lethal dose (human) 5-50 mg/kg, between 7 drops and one teaspoon for 70 kg person (150 lb.). Poisonous if swallowed or inhaled. Human suspected carcinogen.Пожароопасность
Sodium m-arsenite may burn but does not ignite readily. When heated Sodium m-arsenite emits toxic fumes of arsenic and sodium oxide. Slowly converted in solution to arsenates by atmospheric oxygen; in dry state Sodium m-arsenite is decomposed by carbon dioxide.Профиль безопасности
Confirmed human carcinogen. Human poison by ingestion. Experimental poison by ingestion, skin contact, intravenous, intramuscular, and intraperitoneal routes. An experimental teratogen. Experimental reproductive effects. Human mutation data reported. Used as an herbicide and pesticide. When heated to decomposition it emits toxic fumes of arsenic and NazO. See also ARSENIC COMPOUNDS.Возможный контакт
This material is used in manufacturing of arsenical soap for use on skin; treating vines against certain scale diseases; wood preservation; as a reagent in preparation of Methylene iodide; corrosion inhibitor; and for herbicidal and pesticidal purposesПеревозки
UN2027 Sodium arsenite, solid, Hazard Class: 6.1; Labels: 6.1-Poisonous materials. UN1686 Sodium arsenite, aqueous solutions, Hazard Class: 6.1; Labels: 6.1-Poisonous materials.Несовместимости
Chemically active metals. Arsine, a very deadly gas, can be released in the presence of acid, acid mists, or hydrogen gas.Утилизация отходов
The arsenic may be precipitated as calcium arsenite. It should be stored until recycled. In accordance with 40CFR165, follow recommendations for the disposal of pesticides and pesticide containers. Must be disposed properly by following package label directions or by contacting your local or federal environmental control agency, or by contacting your regional EPA office.