SULFATE STANDARD
- русский язык имя
- английское имяSULFATE STANDARD
- CAS №14808-79-8
- CBNumberCB8466491
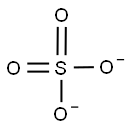
- ФормулаO4S-2
- мольный вес96.06
- EINECS604-622-9
- номер MDLMFCD00145343
- файл Mol14808-79-8.mol
химическое свойство
| плотность | 1.01g/cm3 at 20°C |
| температура хранения | 2-8°C |
| форма | Liquid |
| цвет | Clear colorless |
| РН | 0.8 (20°C in H2O) |
| БРН | 3648446 |
| Справочник по базе данных CAS | 14808-79-8(CAS DataBase Reference) |
| FDA UNII | 7IS9N8KPMG |
| Система регистрации веществ EPA | Sulfate (14808-79-8) |
| UNSPSC Code | 41116116 |
| NACRES | NA.24 |
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)

-
сигнальный язык
предупреждение
-
вредная бумага
H302:Вредно при проглатывании.
H332:Вредно при вдыхании.
SULFATE STANDARD химические свойства, назначение, производство
Описание
The sulfate anion (SO42−) is the stable, oxidized form of sulfur. Sulfate minerals are widely distributed in nature, and most sulfate compounds are readily soluble in water. All sulfate salts are very soluble except for calcium and silver sulfates, which are moderately soluble, and barium, mercury, lead, and strontium sulfates, which are insoluble.It is estimated that about one-half of the river sulfate load arises from mineral weathering and volcanism, and the other half from biochemical and anthropogenic sources. Industrial discharges are another significant source of sulfates. Mine and tailings drainage, smelter emissions, agricultural runoff from fertilized lands, pulp and paper mills, textile mills, tanneries, sulfuric acid production, and metalworking industries are all sources of sulfate-polluted water. Aluminum sulfate (alum) is used as a sedimentation agent for treating drinking water. Copper sulfate is used for controlling algae in raw and public water supplies.
Определение
ChEBI: A sulfur oxoanion obtained by deprotonation of both OH groups of sulfuric acid.Угроза здоровью
The sulfate anion is generally considered nontoxic to animal, aquatic, and plant life. It is an important source of sulfur, an essential nutrient for plants and animals. Sulfates are used as additives in the food industry, and the average daily intake of sulfate from drinking water, air, and food is approximately 500 mg. As examples, some measured sulfate concentrations in beverages are 100–500 mg/L in drinking water, 500 mg/L in coconut milk, 260 mg/L in beer (bitter), 250 mg/L in tomato juice, and 300 mg/L in red wine (FNB 2004). Available data suggest that people acclimate rapidly to the presence of sulfates in their drinking water.No upper limit likely to cause detrimental human health effects has been determined for sulfate in drinking water. However, concentrations of 500–750 mg/L may cause a temporary mild laxative effect, although doses of several thousand milligrams per liter generally do not cause any long-term ill effects. Because of the laxative effects resulting from ingestion of drinking water containing high sulfate levels, the EPA recommends that health authorities be notified of sources of drinking water that contain sulfate concentrations in excess of 500 mg/L.
The presence of sulfate can adversely affect the taste of drinking water, imparting a bitter taste. The lowest taste threshold concentration for sulfate is approximately 250 mg/L as sodium salt, but higher as calcium or magnesium salts (up to 1000 mg/L).
Экологическая судьба
Nearly all natural surface waters and shallow groundwaters contain sulfate anions. Sulfate is commonly found as a prominent component of unpolluted waters and is included among the six major surface and shallow groundwater ions (Na+ , Ca+ , Mg+Cl− , (HCO3)2− , and (SO4)2−), second to bicarbonate as the most abundant anion in most freshwaters. Sulfur is an essential plant and animal nutrient, and sulfate is the most common inorganic form of sulfur in aerobic environments. Sulfate water concentrations that are too low have a detrimental effect on both land and aquatic plant growth.Sulfate is redox sensitive and is bacterially reduced to sulfide ion under anaerobic conditions. Sulfide may be released to the atmosphere as H2S gas or precipitated as insoluble metal sulfides. Oxidation of sulfides returns sulfur to the sulfate form.
Sulfates may be leached from most sedimentary rocks, including shales, with the most appreciable contributions from such sulfate deposits as gypsum (CaSO4·2H2O) and anhydrite (CaSO4 ). The oxidation of sulfur-bearing organic materials can con- tribute sulfates to waters.
SULFATE STANDARD запасные части и сырье
запасной предмет
- Reactive Brilliant Blue M-BR
- 2-[(4-amino-9,10-dihydro-9,10-dioxo-3-sulfo-1-anthracenyl) amino]-4-[[2-(sulfooxy)ethyl]sulfonyl]-Benzoic acid
- REACTIVE BLACK 5
- REACTIVE RED 194
- [2 - [[4 - [(2-циано-4-нитрофенил) азо] фенил] этиламино] этил] триметиламмоний метилсульфат
- Reactive Disperse Scarlet G
- Reactive Turquoise Blue Kn-G
- Reactive Red 180
- Reactive Dark Blue M-2ge
- Reactive Red M-3BE
- Reactive Golden Yellow KM-G
- Silk softener
- Reactive Brilliant Yellow M-7G
- Reactive Red Brown K-B3r
- Reactive Blue BRF
- ribonucleic acid for injection
- Реактивный красный 240
- sec-alkyl sodium sulfate
- РЕАКТИВНЫЙ ФИОЛЕТОВЫЙ 5
- disodium 6-acetamido-4-hydroxy-3-[[4-[[2-(sulphonatooxy)ethyl]sulphonyl]phenyl]azo]naphthalene-2-sulphonate
- Ланоконазол
- Ремазол бриллиантовый синий R
- 3,7-диметил-7-гидроксиоктанал
- Реактивный синий 104
- trisodium [5-acetamido-4-hydroxy-3-[[2-hydroxy-5-[[2-(sulphooxy)ethyl]sulphonyl]phenyl]azo]naphthalene-2,7-disulphonato(5-)]cuprate(3-)
- Reactive Disperse Orange R
- Reactive Black M-2R
- frothing agent K14
- monoethanolamine dodecyl sulfate
- Reactive Light Yellow M-5G
1of8
SULFATE STANDARD поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия |
|---|---|---|---|---|
| +86-29-81139210 +86-18192627656 |
China | 3003 | 58 | |
| +49-7246-3082843 | Germany | 1859 | 58 | |
| +8615350571055 | China | 6087 | 58 | |
| +86-0592-6210733 | China | 32343 | 55 | |
| 021-61119791,13386096464 | China | 3921 | 50 | |
| 400-1166-196 13458535857 |
China | 13350 | 58 | |
| 400-821-8006 | China | 3040 | 75 | |
| 15337241005 13260682861 |
China | 2435 | 58 | |
| 029-81024372 15029046529 |
China | 10010 | 58 | |
| 400-111-6333 | CHINA | 9730 | 58 |